A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Orthotopic Implantation and Peripheral Immune Cell Monitoring in the II-45 Syngeneic Rat Mesothelioma Model
In This Article
Summary
The generation of an orthotopic rat model of pleural malignant mesothelioma by implantation of II-45 mesothelioma cells into the pleural cavity of immune competent rats is presented. A flow cytometric method to analyze seven immune cell subsets in these animals from a 25 µl blood sample is also described.
Abstract
The enormous upsurge of interest in immune-based treatments for cancer such as vaccines and immune checkpoint inhibitors, and increased understanding of the role of the tumor microenvironment in treatment response, collectively point to the need for immune-competent orthotopic models for pre-clinical testing of these new therapies. This paper demonstrates how to establish an orthotopic immune-competent rat model of pleural malignant mesothelioma. Monitoring disease progression in orthotopic models is confounded by the internal location of the tumors. To longitudinally monitor disease progression and its effect on circulating immune cells in this and other rat models of cancer, a single tube flow cytometry assay requiring only 25 µl whole blood is described. This provides accurate quantification of seven immune parameters: total lymphocytes, monocytes and neutrophils, as well as the T-cell subsets CD4 and CD8, B-cells and Natural Killer cells. Different subsets of these parameters are useful in different circumstances and models, with the neutrophil to lymphocyte ratio having the greatest utility for monitoring disease progression in the mesothelioma model. Analyzing circulating immune cell levels using this single tube method may also assist in monitoring the response to immune-based treatments and understanding the underlying mechanisms leading to success or failure of treatment.
Introduction
Malignant mesothelioma (MM) is an aggressive malignancy which arises from transformed cells in the membrane (mesothelium) that lines the lung and abdominal cavities, heart and internal reproductive organs, and is the most common primary tumor of the lung cavity or pleura1,2. Exposure to asbestos fibres accounts for 80% of all MM, and while bans on asbestos use were introduced decades ago in most western countries, its widespread use in the community has left a deadly legacy. The World Health Organization has estimated that 107,000 people worldwide die each year from asbestos related diseases, with mortality rates continuing to increase. A new non-occupational incidence wave is also emerging and there is little understanding of when, and at what level this will peak3.
The majority of people with MM are diagnosed late when systemic chemotherapy represents one of the only viable options4. The most effective chemotherapy and current ‘standard of care’ (pemetrexed together with cisplatin5) was identified over 10 years ago. However failure of this treatment is inevitable and there are no proven second line options, leaving patients with a grim prognosis and median survival of only 12 months2. Therefore, there is an urgent unmet need for more effective treatments. Despite the examination of a number of novel therapies in clinical trials none has resulted in changes in practice. This is due in part to the low (5%) transference of pre-clinical results, generally performed in xenograft mouse models, to the clinical setting6-8. Such models do not faithfully recapitulate the complex aspects of the tumor microenvironment occurring in non-physiological locations, frequently in the absence of a functioning immune system9.
Syngeneic orthotopic models create a significantly more realistic tumor environment than the commonly used subcutaneous xenograft models as the tumors occur in the correct physiological location with an intact immune system10,11. The larger size of the rat enhances its use as a rodent disease model, especially in drug studies where serial blood draws are required to assess treatment response and toxicity12. Furthermore, in models in which monitoring disease progression is difficult due to the location of the tumors (such as in the pleural cavity), the ability to monitor disease progression using factors found in the circulation is extremely attractive. The generation of a syngeneic orthotopic model of pleural mesothelioma using immune-competent rats is described. In addition, an easy and relatively non-invasive method for monitoring pleural disease progression by measuring circulating immune cells is also described.
Protocol
All procedures involving animals were carried out in accordance with the recommendations in the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The protocol for this study was approved by the Royal North Shore Hospital Animal Care and Ethics Committee. Female Fischer 344 rats (F344, 150-200 g) were maintained at the Kearns Facility, Kolling Institute under standard conditions (12 hr light/dark cycles and free access to food and water).
Note: A flow chart for all experimental procedures is presented in Figure 1.
1. Preparation of Cells for Implantation
- Culture the rat mesothelioma II-45 cell line (also known as IL-45; derived by peritoneal introduction of crocidolite asbestos) in RPMI 1640 (RPMI) media supplemented with 10% fetal bovine serum (FBS) and grow in standard conditions (37 °C humidified incubator with 5% CO2). Maintain by passaging and sub-culturing at approximately 1:50 twice a week in a 75 cm2 flask.
- Prepare reagents for cell culture and warm aliquots at 37 °C. Required reagents include serum free RPMI media (SFM), RPMI with 10% FBS, phosphate buffered saline (PBS) and 0.5% trypsin-EDTA.
- Culture cells for implantation to approximately 70-80% confluence. This ensures they are in the linear growth phase.
- Harvest cells by discarding the media, washing once with 5 ml of sterile PBS and then adding 3 ml of 0.5% trypsin-EDTA.
- Return flasks to the incubator for approximately 5 min until all cells become non-adherent.
- Once cells are non-adherent, add 3 ml of RPMI with 10% FBS to inactivate the trypsin. Collect and centrifuge cells at 300 x g for 3 min.
- Wash the cell pellet in 10 ml of SFM and centrifuge again at 300 x g for 3 min.
- Wash cell pellet again with 10 ml of SFM and centrifuge as above.
- Resuspend the cells in 10 ml of SFM and perform a cell count using a hemocytometer or similar instrumentation.
- Dilute cells so that 100 µl contains the amount of cells to be implanted.
Note: Tumor growth has been demonstrated at a dose as low as 100 cells in 100 µl but a standard dose is 500,000 cells in 100 µl. - Prepare sufficient cells in media for the number of rats to be implanted (i.e., 100 µl/rat), plus at least 0.5 ml extra to compensate for losses from priming and the dead volume of the needle.
- Prepare sufficient SFM (without cells) to be implanted into control rats (i.e., 100 µl/rat), plus at least 0.5 ml extra.
Note: The cells and SFM are now ready for implantation. They should be kept at 37 oC and implanted within 2 hr of harvesting to maintain viability.
2. In vivo Implantation of Cells
- Place the F344 rat (> 13 weeks of age) into the induction chamber and anesthetize using 1.4% isoflurane inhalation (or the method in use in the facility). Once the rat appears to be asleep move it from the chamber to a nose cone (with 1.4% isoflurane flowing), place it on its back with chest facing up (ventral view). This allows the internal organs to settle away from the chest cavity. Check reflexes according to institutional protocols to ensure the rat is fully anesthetized.
- Shave the right regio costalis (chest) area to remove the fur.
- Clean the shaven area with 80% v/v ethanol.
- Identify the injection site: on the right side, find the 2nd gland starting cranial. The injection site is 0.5 cm proximal to this, in between the 3rd and 4th rib from the caudal end of the rib cage. (Figure 2A).
- Gently mix the II-45 cells to resuspend. Slowly draw the cell suspension (or SFM for control rats) into a 1 ml syringe without a needle attached. If a needle is attached for the drawing up of cells there is the potential for cells to grow along the needle injection line. Attach a 23G x 1¼ needle. Prime the needle and remove any air bubbles.
- Once the syringe and needle are primed, place a 20 mm long and 5 mm diameter spacer over the needle shaft. This is used to prevent the needle from penetrating too deeply into the pleural cavity during the injection. Approximately 5 mm-12 mm of exposed needle is sufficient for penetration through the ribs without damaging any organs.
- Slowly insert the needle between the ribs, draw back on the syringe to ensure a blood vessel has not been punctured (no blood should appear in the syringe) then inject 100 μl cells or SFM. (Figure 2B).
- Remove the needle and gently roll the rat from side to side to spread cells in the chest cavity.
- Place the rat into a cage and check for recovery. The rat should be awake within 1 min and starting to move around.
- Repeat for each rat using a new needle. Reusing the same needle will result in cell growth along the injection line of the needle.
- Monitor the well-being of the animals daily.
- Euthanize animals at ethically defined endpoints as governed by the institutional animal ethics committee. The ethical endpoints for the rats in these experiments were weight loss of greater than 10% or labored breathing.
3. Tail Vein Blood Collection
- If blood is to be collected immediately post-cell implantation, keep the rat anesthetized. If sampling blood at another time point, anesthetize the rat using 1.4% isoflurane inhalation. Check reflexes according to institutional protocols to ensure the rat is fully anaesthetized.
- Place the rat onto its side and locate a lateral tail vein.
- Sterilize the tail with 80% ethanol and label a 0.5 ml EDTA collection tube.
- To collect blood, always start at the caudal end of the tail (approximately one third of the way along). This allows further attempts closer to the cranial end of the tail in case the first attempt is unsuccessful. Never resample caudally as this can cause a blood clot.
- Position a 23G x 1¼ needle parallel to the lateral vein and slide it into the vein at a shallow angle so it penetrates approximately 10 mm (Figure 3A).
- Note: If the vein has been successfully punctured blood will be visible in the attachment end of the needle (Figure 3B).
- A drop of blood will form on the tail at the site of the needle puncture. Collect this blood using a pipette and transfer into the labelled 0.5 ml (or smaller) EDTA collection tube. For the immune cell assay 25 μl is sufficient. Apply gauze with pressure to puncture site until bleeding stops.
- Flick the blood tube to mix the blood and EDTA to prevent clotting. Keep the time between blood collection and mixing with the EDTA as short as possible to prevent clotting.
- When collecting blood from multiple rats store EDTA-blood samples in a rack at RT until analysis. Process blood within 2 hr of collection.
4. Sample Preparation for Immune Cell Profiling Using the Bead-based Method
Note: This single platform method relies on using commercially available absolute counting tubes that have a known number of beads for each sample. These tubes contain lyophilized pellets that dissolve during sample preparation, releasing the beads. The beads are fluorescently labelled and by gating on the bead population, absolute counts can be calculated.
- Ensure the EDTA whole blood sample is well mixed by placing it on a slow rotary mixer for several min. Label one absolute counting tube for each sample. A pellet containing the beads should be visible underneath the metal bead holder at the bottom of the tube.
- Transfer 25 µl of EDTA whole blood into a labelled absolute counting tube. The bead pellet will dissolve upon addition of the blood.
- To each tube add 20 µl of anti-rat T/B/Natural Killer (NK) cell cocktail, 10 µl of anti-rat CD8a PE, 10 µl of anti-rat CD4 (domain 1) FITC and 10 µl of anti-rat CD45 PE/Cy7 (Figure 4A). Fluorophores are defined in Table 1.
- Centrifuge the tube briefly (300 x g) to ensure the antibodies and cells are in the bottom of the tube and not stuck to the side of the tube. Vortex to mix and incubate for 15 min at RT.
- To lyse red blood cells add 400 µl of 10 mM Tris, 0.15 M ammonium chloride buffer (pH 7.5) and vortex to mix. Lysis is complete when the sample appears translucent and not cloudy (Figures 4B and C). Failure to lyse the sample completely will lead to increased background and falsely elevated counts when analyzing by flow cytometry.
5. Flow Cytometric Processing of Samples
Note: Perform on a 4 color flow cytometer.
- Open the software in acquisition mode and a new template with 8 plots as depicted in Figure 5.
- Adjust instrument settings to those listed in Table 1 and set up gate R1 (FITC [FL-1] versus APC [FL-4]), Figure 5Ai) to count the fluorescent beads. The other gates are not as important at this acquisition stage but will be required for analysis. The absolute counting beads used in this protocol contain fluorescent dyes and can be detected in any channel although are weakest in the blue channel.
- Using a prepared control blood sample, vortex and then load onto the cytometer and run at a low speed (12 µl/min) on setup mode so data acquisition gates can be adjusted.
- Set the acquisition to collect 10,000 events in the R1 bead gate.
- Set up a folder to record data and set file number and label sample file in acquisition menu.
- Load the sample to be analyzed onto the cytometer and set the flow rate to medium (35 µl/min). Run each sample at the same flow rate. The flow rate may need to be varied to low (12 µl/min) or high (60 µl/min), but medium is generally appropriate. At this rate it takes approximately 90 to 120 sec to acquire 10,000 bead events for each sample.
- Once the sample is loaded watch the scatter plots to make sure events are appearing in the R1 bead gate. Initially there can be some instability in the sample pressure causing drift in the scatter plots. Wait for this to stabilize.
- Once stabilized, click on acquire and allow sample to run. Once the cytometer has finished acquiring 10,000 bead events in R1 the cytometer will stop acquiring and save all data.
- Remove the sample and discard flow tube. The cytometer is now ready for the next sample. Run all samples and then proceed to analysis mode.
6. Immune Cell Analysis
Note: Gating strategies and Boolean algebra are used to define each cell population. Boolean algebra is a logic based analysis method that allows for multiple operations in a single definition. The analysis software of the flow cytometer (e.g., BD CELLQuest) allows for the use of Boolean algebra. The equations are used to actively account for the significant negative reactivity that assists in defining the cell to more specifically identify each cell population. ‘Regions’ are used to define a ‘gate’. Regions define a 2 dimensional space whereas gates can be composed of numerous regions connected by algebraic operators (+, *,-, defined in Table 2).
- Switch the software to analysis mode. An analysis template should be generated to match Figure 5 with the plots and gates shown.
- Analyze each individual file (i.e., each individual sample) separately. Set up gates R1 through to R9 and then set up the algorithms for each cell type as defined in Table 2 (also shown in Figure 5) .
- Use the cell statistics counter to calculate individual cell populations defined by gates and algorithms (Table 2 and Figure 5). The algorithms will adjust cell numbers automatically in the cell statistics counter.
- Calculate cell subsets using the following equation:
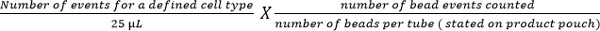
Note: Number of cell events counted (e.g., CD4 T cell events) is enumerated using the above equation to give cell number per µl of blood. Examples are shown in Figure 5.
Results
The method used in this paper for the generation of an orthotopic model of pleural mesothelioma using II-45 cells resulted in animals succumbing to mesothelioma in a reproducible and rapid timeframe, with no rats dying due to the implantation method. Titration of the number of cells implanted determined that 1x 103 cells was the minimum number required for a fully penetrant model (100% engraftment). The different number of cells implanted in the rats changed the time course of the disease without appearing to ...
Discussion
This paper details a method for the generation of a rat syngeneic orthotopic model of pleural mesothelioma and a simple method for monitoring disease progression through longitudinal blood sampling.
The II-45 model was developed by exposing Fischer 344 rats to asbestos fibers13. Although this exposure represents the true dynamics of host-asbestos-immune system interactions for mesothelioma pathogenesis, it has a long lag time (taking years to generate) and can be dangerous for the r...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The syngeneic rat mesothelioma II-45 cell line was a kind gift from A/Prof. Emanuela Felley-Bosco, Zurich University.
Materials
| Name | Company | Catalog Number | Comments |
| EDTA Collection tube (0.5 ml) | Greiner Bio One GmbH | 450480 | |
| Rat T/B/NK Cell cocktail | BD Pharmingen | 558509 | anti-Rat CD3 APC (IgM clone 1F4), anti-Rat CD45RA Fitc (IgG1 clone OX-33), anti-Rat CD161a PE (IgG1 Clone 10/78) |
| anti-RAT CD8a PE | Biolegend | 200608 | (IgG1vClone G28) |
| anti-Rat CD4 FITC (Domain 1) | Biolegend | 203406 | (IgG1 Clone OX-38) |
| anti-Rat CD45 PE/Cy7 | Biolegend | 202214 | (IgG1 Clone OX-1) |
| TruCount Tubes | Becton Dickinson | 340334 | Box of 50 absolute counting tubes |
| RPMI 1640 media | Life Technologies | 11875-119 | |
| foetal bovine serum (FBS) | Scientifix | FBS500-S (lot# 010101-1) | |
| trypsin-EDTA | Life Technologies | 15400-054 | |
| PBS tablets | Medicago AB | 09-9400-100 | |
| 23Gx1¼ Needle | Becton Dickinson | 302008 | |
| 1 ml Syringe | Becton Dickinson | 302 100 | |
| Fischer 344 Rat | Animal Resources Centre, Perth Australia | F344 | |
| I.S.O (Isoflurane USP) | Veterinary Companys Australia (VCA) | B7058 | |
| II-45 Rat Mesothelioma line | Zurich University | Note: The cell line was given as a gift and is not commercially available at the ATCC | |
| FACSCalibur 4 color | Becton Dickinson | 342975 | |
| TRIS-HCL | SIGMA | T3253 | |
| Ammonium Chloride | SIGMA | 9718 | |
| Anaesthetic Machine (The stinger) | Advanced Anaesthesia specialists | #00449 |
References
- Kao, S. C., et al. Malignant mesothelioma. Intern Med J. 40 (11), 742-7450 (2010).
- Zucali, P. A., et al. Advances in the biology of malignant pleural mesothelioma. Cancer Treat Rev. 37 (7), 543-558 (2011).
- Olsen, N. J., et al. Increasing incidence of malignant mesothelioma after exposure to asbestos during home maintenance and renovation. Med J Aust. 195 (5), 271-274 (2011).
- Zucali, P. A., et al. Thymidylate synthase and excision repair cross-complementing group-1 as predictors of responsiveness in mesothelioma patients treated with pemetrexed/carboplatin. Clin Cancer Res. 17 (8), 2581-2590 (2011).
- Vogelzang, N. J., et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 21 (14), 2636-2644 (2003).
- Lowenstein, P. R., Castro, M. G. Uncertainty in the translation of preclinical experiments to clinical trials. Why do most phase III clinical trials fail?. Curr Gene Ther. 9 (5), 368-374 (2009).
- Kamb, A. What's wrong with our cancer models. Nat Rev Drug Discov. 4 (2), 161-165 (2005).
- Yakisich, J. S. An Algorithm for the Preclinical Screening of Anticancer Drugs Effective against Brain Tumors. ISRN Pharmacol. 2012, 513580 (2012).
- Basu, D., Herlyn, M. Defining microenvironments within mouse models that enhance tumor aggressiveness. Cancer Biol Ther. 8 (4), 380-381 (2009).
- Abolhassani, M., et al. Screening of well-established drugs targeting cancer metabolism: reproducibility of the efficacy of a highly effective drug combination in mice. Invest New Drugs. 4 (4), 1331-1342 (2011).
- Hudson, A. L., et al. Establishing a panel of chemo-resistant mesothelioma models for investigating chemo-resistance and identifying new treatments for mesothelioma. Sci Rep. 4, 6152 (2014).
- Iannaccone, P. M., Jacob, H. J. Rats!. Dis Model Mech. 2 (5-6), 206-210 (2009).
- Craighead, J. E., et al. Characteristics of tumors and tumor cells cultured from experimental asbestos-induced mesotheliomas in rats. Am J Pathol. 129 (3), 448-462 (1987).
- Hunter, S. D., et al. Lymphocyte subset analysis by Boolean algebra: a phenotypic approach using a cocktail of 5 antibodies and 3 color immunofluorescence. Cytometry. 15 (3), 258-266 (1994).
- Brando, B., et al. Cytofluorometric methods for assessing absolute numbers of cell subsets in blood. European Working Group on Clinical Cell Analysis. Cytometry. 42 (6), 327-346 (2000).
- Schnizlein-Bick, C. T., et al. Evaluation of TruCount absolute-count tubes for determining CD4 and CD8 cell numbers in human immunodeficiency virus-positive adults. Clin Diagn Lab Immunol. 7 (3), 336-343 (2000).
- Gajkowska, A., et al. Flow cytometric enumeration of CD34+ hematopoietic stem and progenitor cells in leukapheresis product and bone marrow for clinical transplantation: a comparison of three methods. Folia Histochem Cytobiol. 44 (1), 53-60 (2006).
- Hanahan, D., Weinberg, R. A. Hallmarks of cancer: the next generation. Cell. 144 (5), 646-674 (2011).
- Kao, S. C., et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res. 16 (23), 5805-5813 (2010).
- Kao, S. C., et al. Validation of prognostic factors in malignant pleural mesothelioma: a retrospective analysis of data from patients seeking compensation from the New South Wales dust diseases board. Clin Lung Cancer. 14 (1), 70-77 (2013).
- Burt, B. M., et al. Circulating and tumor-infiltrating myeloid cells predict survival in human pleural mesothelioma. Cancer. 117 (22), 5234-5244 (2011).
- Weir, C., et al. Streptavidin: a novel immunostimulant for the selection and delivery of autologous and syngeneic tumor vaccines. Cancer Immunol Res. 2 (5), 469-479 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved