鼠后肢长骨解剖和骨髓隔离
In This Article
Summary
Here we present a protocol for the dissection of hind limb long bones (femurs and tibiae) from the laboratory mouse. We further describe a rapid technique for bone marrow isolation from these bones that utilizes centrifugation for removal of bone marrow from the bone marrow space.
Abstract
Investigation of the bone and the bone marrow is critical in many research fields including basic bone biology, immunology, hematology, cancer metastasis, biomechanics, and stem cell biology. Despite the importance of the bone in healthy and pathologic states, however, it is a largely under-researched organ due to lack of specialized knowledge of bone dissection and bone marrow isolation. Mice are a common model organism to study effects on bone and bone marrow, necessitating a standardized and efficient method for long bone dissection and bone marrow isolation for processing of large experimental cohorts. We describe a straightforward dissection procedure for the removal of the femur and tibia that is suitable for downstream applications, including but not limited to histomorphologic analysis and strength testing. In addition, we outline a rapid procedure for isolation of bone marrow from the long bones via centrifugation with limited handling time, ideal for cell sorting, primary cell culture, or DNA, RNA, and protein extraction. The protocol is streamlined for rapid processing of samples to limit experimental error, and is standardized to minimize user-to-user variability.
Introduction
The study of long bones and the cells of the bone marrow is central to a myriad of research disciplines, including, but not limited to, bone biology, cancer biology, immunology, hematology, and biomechanics. The bone is a highly dynamic organ that together with the cartilage forms the skeleton to provide mechanical support against loading and protection of the internal organs. In addition, the mineral components of bone are a storage sink for the critical signaling molecules calcium and phosphorus, as well as other factors1. Finally, bones house the bone marrow and, together with metabolically active bone forming osteoblasts and bone resorbing osteoclasts, provide the stem cell niche necessary for the maintenance of hematopoietic and lymphoid cell populations.
Bone and bone marrow are affected in many disorders, often leading to bone marrow dysfunction, severe bone pain, and pathologic fracture. Bone is a common site of metastasis in many solid tumors, most notably breast cancer and prostate cancer, where tumor cells directly engage the bone marrow niche to initiate the vicious cycle of bone metastasis and displace hematopoietic stem cells2,3. Hematopoietic malignancies including myeloma and leukemia are characterized by bone marrow dysfunction as well as deregulation of healthy bone remodeling1. Other non-malignant skeletal disorders are also active areas of research, such as osteoarthritis, osteoporosis, scoliosis, and rickets. Even in an otherwise healthy individual, biomechanical failure in a bone leads to a painful fracture. All of these disorders represent active areas of research with the goal of identifying new preventative measures and treatment regimens to reduce morbidity and mortality.
To research the plethora of roles of the bone and the bone marrow, both under physiologic and pathologic conditions, it is critical for researchers to have a simple and efficient standardized method for dissection of the mouse long bones for rapid processing of large in vivo experiments. The dissection protocol outlined here is suitable for all long bone analyses including ex vivo imaging, histology, histomorphometry, and strength testing, among others. Similarly, a standardized bone marrow isolation method with high bone marrow cell recovery and low inter-user variability is important for experimental analysis such as fluorescence-activated cell sorting (FACS) or quantitative PCR (qPCR) as well as downstream applications such as primary cell culture of bone marrow cells.
Protocol
所有的动物工作按照指南中概述了美国国立卫生研究院的实验动物的护理和使用的建议被批准的机构动物护理和使用委员会。
1.后肢长骨解剖
- 安乐死按照机构准则鼠标。
- 鼠标在仰卧位置定位并通过踝关节下方的小鼠爪垫钉扎所有四条腿贴上。
- 喷鼠标用70%乙醇,充分浇熄腿部。
- 做一个小切口,中线右侧下腹部,只是臀部上方。
- 向下延伸的腿和过去的踝关节的切口。
- 拉回皮肤和切割锚固到股骨近端股四头肌以暴露股骨的前侧并从腿部引脚输出,将所述销从所述板成45度角。
- 随着BLAD对股骨的后侧的剪刀电子,切腿筋从膝关节程。
- 拉回皮肤和锚定在股骨的近端,以暴露该股骨的后侧并从腿销从腿筋肌肉,将销从所述板成45度角。
- 与镊子,保持股骨的远端,在膝盖关节的上方。引导朝向所述髋关节的股骨干的任一侧上的剪刀刀片,小心不要切入股骨本身。
- 到达股骨头,用剪刀轻轻打开后表示,拧剪刀用剪刀直接在股骨头移动的顶部叶片打乱股骨,小心不要捕捉股骨头下方的骨头。
- 把握股骨干与镊子的顶端,切割软组织从股骨头远离从髋臼松开。
- 拉动整个腿骨,包括股骨,膝,和胫骨,并远离身体,小心地切去结缔组织和肌肉的腿连接到皮肤上。
- 透支踝关节和再次使用剪刀扭转运动脱臼胫骨。
- 抓胫骨的远端,小心不要切断筋,并从主体和销板远离拉胫骨。
- 切断的任何剩余结缔组织在膝盖附接长骨的鼠标。
- 删除的任何其他肌肉或附到股骨和胫骨的结缔组织。
- 对于需要骨的任何应用程序保持不动(组织学,组织形态计量学,生物力学测试, 等 ),继续进行标准的内部协议(如在4-7)。为了分离骨髓,进行第2节。
2.长骨准备骨髓隔离
- 使用镊子,把握股骨髌骨背向一第二近端(股骨头)下来。
- 透支膝关节,并使用剪刀扭转运动打乱了胫骨和股骨。
- 切的任何结缔组织保持股骨和胫骨在一起。
- 使用镊子,把握股骨与前侧背向和近端(股骨头端)向下。
- 股骨轴引导剪刀到髁。
- 轻轻转动剪刀来回除去髁,髌骨和骨骺揭露干骺端。
- 删除的任何其他肌肉或使用镊子,剪刀和的Kimwipes附着于股骨结缔组织。
- 使用镊子,把握胫骨与前侧背向和远端(脚踝端)向下。
- 如果骨骺胫骨完好,引导剪了胫骨的髁。
- 轻轻转动剪刀来回去除髁骨骺揭露metaphysis。
- 删除的任何其他肌肉或使用镊子,剪刀和的Kimwipes附着在胫骨的结缔组织。
3.骨髓隔离
- 通过0.5 ml离心管的底部推18G的针。
- 将长骨(最多2股骨和胫骨2)入管,膝降到底端,然后合上盖子。
- 窝在一个1.5 ml离心管0.5 ml离心管。
- 离心机≥10,000XG嵌套管在15秒的离心。
- 验证骨髓已通过目测纺出的骨头。骨头应该出现白色应该有较大的管大视觉沉淀。
- 丢弃的骨头0.5 ml离心管。
- 暂停在适当溶液骨髓( 例如 ,PBS,培养基,FACS缓冲液),并继续进行与实验协议(DNA,RNA或蛋白质分离,FACS分析,或原代细胞培养物)。
Representative Results
这里所描述的协议为鼠标股骨和胫骨以最小到骨组织损伤的快速解剖优化。该技术是适合于许多下游的分析,包括生物力学研究中,组织形态计量学( 图1A - B)和组织学( 图1C)4,7。代表histomophometric micoCT三维重建( 图1A - B)中表明,两者的松质骨和皮质壳被维持,允许骨组织形态计量学,包括骨小梁数,厚度和间距的标准化结构参数的准确定量;骨量;和皮质厚度等措施8之一。该代表组织切片显示了H&E染色福尔马林固定和脱钙胫骨( 图1C)。图像展示两个钙化b的完整性之一,细胞的骨髓进行组织学分析。
骨髓分离过程保留骨髓空间的无菌性,具有低的处理,以减少污染,并且不需要长骨的切割,从而减少骨髓产量的损失。这骨髓是适合于许多下游应用,包括流式细胞仪5和PCR分析。此外,此过程可用于分离骨髓细胞,包括破骨细胞和成骨细胞( 图2A - B)的原代培养骨髓4,6。
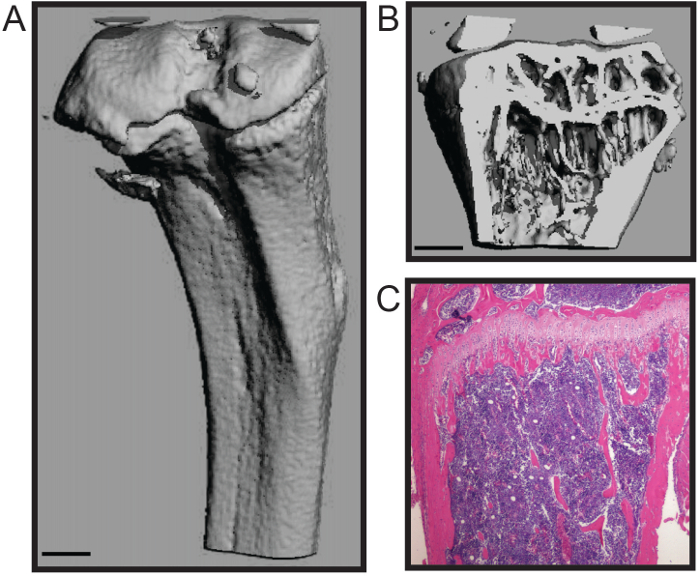
显示(A)外皮质外壳和鼠标胫骨图1.组织形态学和鼠标的长骨的组织学分析。三维显微重建(B 仲>)骨小梁(比例尺= 0.5毫米)。一个脱钙和切片胫骨(4倍),(三)组织学H&E染色。图片凯瑟琳Weilbaecher,医学院,美国华盛顿大学医学院提供。 请点击此处查看该图的放大版本。

图2.原发性骨髓细胞培养的破骨细胞和成骨细胞分化的影响。(A)TRAP染色后7天破骨媒体(4倍)多核破骨细胞。 (B)碱性磷酸酶(紫色),成骨细胞和茜素红(红色)染色后对成骨媒体21天矿化。凯瑟琳Weilbaecher图像礼貌,医药,美国华盛顿大学医学院。尔斯/ ftp_upload / 53936 / 53936fig2large.jpg“目标=”_空白“>点击此处查看该图的放大版本。
Discussion
We present a simple and efficient method for removal of mouse hind long bones and subsequent bone marrow isolation. This method maintains the high structural and cellular integrity of the bones and bone marrow and has low handling time, minimizing the likelihood of user-induced fracture or bone scoring that may influence downstream analyses. In addition, the centrifugation method for isolating bone marrow does not require cutting the bone to expose the bone marrow space or fluid to flush the bone marrow, reducing potential points of contamination. Moreover, the centrifuge technique is relatively high-throughput with lower hands-on time than other methods, thus reducing processing time.
High variation is inherent to in vivo mouse studies due to high mouse-to-mouse phenotypic variation. In order to maximize the research impact of expensive and labor-intensive mouse studies, it is critical to minimize technical experimental error9,10. Time from animal sacrifice to downstream analysis or tissue fixation introduces experimental variation that may overcome subtle changes and reduce large differences between groups. Therefore, rapid processing of samples is essential for accurate data analysis. The long bone dissection and bone marrow isolation techniques described here are optimized for rapid processing of animals and samples to reduce technical variation.
This protocol can be widely applied to many research fields, including investigation of the bone tissue itself or interrogation of the cells of the bone marrow. In addition, this straightforward approach to long bone dissection will enable researchers in related fields to directly interrogate bone contributions in order to expand our knowledge of bone marrow dysfunction in otherwise understudied pathologies.
Acknowledgements
This work was supported by NCI grant nos. U54CA143803, CA163124, CA093900, and CA143055 to K.J.P. The authors thank the current and past members of the Weilbaecher lab, especially Katherine Weilbaecher, Michelle Hurchla, and Hongju Deng, and members of the Brady Urological Institute, especially members of the Pienta laboratory for critical reading of the manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| pinboard | |||
| pins | |||
| 70% ethanol | |||
| dissection sissors | |||
| dissection forceps | |||
| Kimwipes | Kimberly-Clark | 34120 | |
| 16 guage needle | |||
| 1.5 ml microcentrifuge tube | |||
| 0.5 ml microcentrifuge tube | |||
| microcentrifuge |
References
- McHayleh, W. M., Ellerman, J., Roodman, G. D. Ch. 80. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. , 379-381 (2008).
- Weilbaecher, K. N., Guise, T. A., McCauley, L. K. Cancer to bone: a fatal attraction. Nat Rev Cancer. 11 (6), 411-425 (2011).
- Pedersen, E. A., Shiozawa, Y., Pienta, K. J., Taichman, R. S. The prostate cancer bone marrow niche: more than just 'fertile soil. Asian J Androl. 14 (3), 423-427 (2012).
- Amend, S. R., et al. Thrombospondin-1 regulates bone homeostasis through effects on bone matrix integrity and nitric oxide signaling in osteoclasts. J Bone Miner Res. 30 (1), 106-115 (2015).
- Hurchla, M. A., et al. The epoxyketone-based proteasome inhibitors carfilzomib and orally bioavailable oprozomib have anti-resorptive and bone-anabolic activity in addition to anti-myeloma effects. Leukemia. 27 (2), 430-440 (2013).
- Rauch, D. A., et al. The ARF tumor suppressor regulates bone remodeling and osteosarcoma development in mice. PLoS One. 5 (12), e15755 (2010).
- Su, X., et al. The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. J Clin Invest. 122 (10), 3579-3592 (2012).
- Dempster, D. W., et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 28 (1), 2-17 (2013).
- Begley, C. G., Ellis, L. M. Drug development: Raise standards for preclinical cancer research. Nature. 483 (7391), 531-533 (2012).
- Festing, M. F., Altman, D. G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 43 (4), 244-258 (2002).
Explore More Articles
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved