Murine Hind Limb os long Dissection et moelle osseuse Isolation
In This Article
Summary
Here we present a protocol for the dissection of hind limb long bones (femurs and tibiae) from the laboratory mouse. We further describe a rapid technique for bone marrow isolation from these bones that utilizes centrifugation for removal of bone marrow from the bone marrow space.
Abstract
Investigation of the bone and the bone marrow is critical in many research fields including basic bone biology, immunology, hematology, cancer metastasis, biomechanics, and stem cell biology. Despite the importance of the bone in healthy and pathologic states, however, it is a largely under-researched organ due to lack of specialized knowledge of bone dissection and bone marrow isolation. Mice are a common model organism to study effects on bone and bone marrow, necessitating a standardized and efficient method for long bone dissection and bone marrow isolation for processing of large experimental cohorts. We describe a straightforward dissection procedure for the removal of the femur and tibia that is suitable for downstream applications, including but not limited to histomorphologic analysis and strength testing. In addition, we outline a rapid procedure for isolation of bone marrow from the long bones via centrifugation with limited handling time, ideal for cell sorting, primary cell culture, or DNA, RNA, and protein extraction. The protocol is streamlined for rapid processing of samples to limit experimental error, and is standardized to minimize user-to-user variability.
Introduction
The study of long bones and the cells of the bone marrow is central to a myriad of research disciplines, including, but not limited to, bone biology, cancer biology, immunology, hematology, and biomechanics. The bone is a highly dynamic organ that together with the cartilage forms the skeleton to provide mechanical support against loading and protection of the internal organs. In addition, the mineral components of bone are a storage sink for the critical signaling molecules calcium and phosphorus, as well as other factors1. Finally, bones house the bone marrow and, together with metabolically active bone forming osteoblasts and bone resorbing osteoclasts, provide the stem cell niche necessary for the maintenance of hematopoietic and lymphoid cell populations.
Bone and bone marrow are affected in many disorders, often leading to bone marrow dysfunction, severe bone pain, and pathologic fracture. Bone is a common site of metastasis in many solid tumors, most notably breast cancer and prostate cancer, where tumor cells directly engage the bone marrow niche to initiate the vicious cycle of bone metastasis and displace hematopoietic stem cells2,3. Hematopoietic malignancies including myeloma and leukemia are characterized by bone marrow dysfunction as well as deregulation of healthy bone remodeling1. Other non-malignant skeletal disorders are also active areas of research, such as osteoarthritis, osteoporosis, scoliosis, and rickets. Even in an otherwise healthy individual, biomechanical failure in a bone leads to a painful fracture. All of these disorders represent active areas of research with the goal of identifying new preventative measures and treatment regimens to reduce morbidity and mortality.
To research the plethora of roles of the bone and the bone marrow, both under physiologic and pathologic conditions, it is critical for researchers to have a simple and efficient standardized method for dissection of the mouse long bones for rapid processing of large in vivo experiments. The dissection protocol outlined here is suitable for all long bone analyses including ex vivo imaging, histology, histomorphometry, and strength testing, among others. Similarly, a standardized bone marrow isolation method with high bone marrow cell recovery and low inter-user variability is important for experimental analysis such as fluorescence-activated cell sorting (FACS) or quantitative PCR (qPCR) as well as downstream applications such as primary cell culture of bone marrow cells.
Protocol
Tous les travaux des animaux a été approuvé par l'Institutional Animal Care et utilisation Comité conformément aux recommandations énoncées dans le Guide pour le soin et l'utilisation des animaux de laboratoire des National Institutes of Health.
Dissection 1. Hind Limb os long
- Euthanasier la souris conformément aux directives institutionnelles.
- Placez la souris dans une position couchée et apposer en épinglant les quatre pattes à travers les coussinets des pattes de souris ci-dessous l'articulation de la cheville.
- Pulvériser la souris avec 70% d'éthanol, arrosant abondamment les jambes.
- Faire une petite incision à la droite de la ligne médiane dans le bas ventre, juste au-dessus de la hanche.
- Prolongez l'incision dans la jambe et au-delà de l'articulation de la cheville.
- Tirez la peau et couper le muscle quadriceps ancré à l'extrémité proximale du fémur pour exposer la face antérieure du fémur et la broche à partir de la jambe, en plaçant la tige à un angle de 45 degrés par rapport à la carte.
- Avec le blade des ciseaux contre la face postérieure du fémur, couper les tendons du jarret loin de l'articulation du genou.
- Tirez la peau et les muscles ischio-jambiers ancrés à l'extrémité proximale du fémur pour exposer la face postérieure du fémur et la broche à partir de la jambe, en plaçant la tige à un angle de 45 degrés par rapport à la carte.
- Avec la pince, prise de l'extrémité distale du fémur, juste au-dessus de l'articulation du genou. Guide des lames des ciseaux de chaque côté de la diaphyse fémorale vers l'articulation de la hanche, en faisant attention à ne pas couper dans le fémur lui-même.
- Après avoir atteint la tête fémorale, indiquée par les ciseaux ouverture légèrement, tournez les ciseaux avec la lame supérieure des ciseaux mobiles directement sur la tête fémorale à disloquer le fémur, en faisant attention de ne pas casser l'os en dessous de la tête fémorale.
- Saisissez le haut de la diaphyse fémorale avec la pince, couper les tissus mous loin de la tête fémorale pour le libérer de l'acétabulum.
- Tirez l'ensemble de l'os de la jambe, y comprisfémur, le genou et le tibia, le haut et loin du corps, découpant soigneusement le tissu conjonctif et les muscles de la jambe de liaison à la peau.
- Éparpille l'articulation de la cheville et de nouveau utiliser les ciseaux dans un mouvement de torsion pour disloquer le tibia.
- Saisissant l'extrémité distale du tibia, en prenant soin de ne pas couper les tendons, tirez le tibia et loin du corps et la carte de la broche.
- Couper tout tissu conjonctif restant fixer l'os long à la souris au niveau du genou.
- Enlever tout muscle ou du tissu conjonctif supplémentaire attaché au fémur et au tibia.
- Pour toutes les applications qui nécessitent l'os restent intacts (histologie, histomorphométrie, tests biomécaniques, etc.), Procéder à des protocoles internes standards (comme dans 4-7). Pour isoler la moelle osseuse, passez à la section 2.
2. Préparation à long os pour moelle osseuse Isolation
- Utilisation de la pince, saisir le fémur avec la rotule opposée unee l'extrémité proximale (tête fémorale) vers le bas.
- Éparpille l'articulation du genou et d'utiliser les ciseaux dans un mouvement de torsion pour disloquer le tibia et le fémur.
- Couper tout tissu conjonctif tenant le fémur et le tibia ensemble.
- Utilisation de la pince, saisir le fémur avec la face antérieure opposée et l'extrémité proximale (extrémité de la tête fémorale) vers le bas.
- Guide des ciseaux jusqu'à la diaphyse fémorale aux condyles.
- Tournez doucement les ciseaux et en arrière pour enlever les condyles, la rotule, et l'épiphyse pour exposer la métaphyse.
- Retirez tout musculaire supplémentaire ou de tissu conjonctif attaché au fémur à l'aide des pinces, des ciseaux, et Kimwipes.
- À l'aide de la pince, saisir le tibia avec la face antérieure tournée à l'opposé et l'extrémité distale (extrémité de la cheville) vers le bas.
- Si l'épiphyse tibiale est intact, guider les ciseaux jusqu'à l'arbre du tibia aux condyles.
- Tournez doucement les ciseaux et en arrière pour enlever les condyles et épiphyse pour exposer la métaphyse.
- Retirez tout musculaire supplémentaire ou de tissu conjonctif attaché au tibia à l'aide des pinces, des ciseaux, et Kimwipes.
3. Bone Marrow Isolation
- Pousser une aiguille de 18 G à travers le fond d'un tube à microcentrifugation de 0,5 ml.
- Placez les os longs (maximum de 2 fémurs et 2 tibias) dans le tube, le genou-end end vers le bas vers le bas et fermer le couvercle.
- Nest le tube de 0,5 ml de microcentrifugation dans un tube de 1,5 ml.
- Centrifuger les tubes emboîtés à ≥10,000 xg dans une microcentrifugeuse pendant 15 sec.
- Vérifier que la moelle osseuse a été filé à des os par inspection visuelle. Les os doivent apparaître blanc et il devrait y avoir un grand culot visuel dans le plus grand tube.
- Jeter le tube de 0,5 ml de microcentrifugation avec les os.
- Suspendre la moelle osseuse dans une solution appropriée (par exemple, PBS, milieux de culture, le tampon FACS) et procéder à protocole expérimental (ADN, ARN, ou l' isolement de la protéine,l'analyse par FACS, ou une culture cellulaire primaire).
Representative Results
Le protocole décrit ici est optimisé pour la dissection rapide du fémur de la souris et le tibia avec un minimum de dommages au tissu osseux. Cette technique convient à un certain nombre d'analyses en aval, y compris des études de biomécanique, histomorphométrie (Figure 1A - B), et l' histologie (figure 1C) 4,7. Le histomophometric représentant de la reconstruction micoCT 3D (Figure 1A - B) montre que les deux os spongieux et la coque corticale sont maintenus qui permet une quantification précise des paramètres structurels normalisés pour histomorphométrie osseuse, y compris le numéro trabéculaire, l' épaisseur et l' espacement; volume osseux; et l' épaisseur corticale, entre autres 8. La section histologiques représentant montre un formaline fixe et décalcifiée tibia H & E tachée (figure 1C). L'image montre l'intégrité de la b calcifiéeune et cellulaire de la moelle osseuse pour l'analyse histologique.
La procédure d'isolement de la moelle osseuse préserve la stérilité de l'espace médullaire, a une faible manipulation pour réduire la contamination, et ne nécessite pas la coupe de l'os long, réduisant ainsi la perte de rendement de la moelle osseuse. Cette moelle osseuse est adapté à de nombreuses applications en aval, y compris de cytométrie de flux 5 et PCR analyses. En outre, cette procédure peut être utilisée pour isoler la moelle osseuse pour la culture de cellules primaires de cellules de moelle osseuse, y compris les ostéoclastes et les ostéoblastes (figure 2A - B) 4,6.
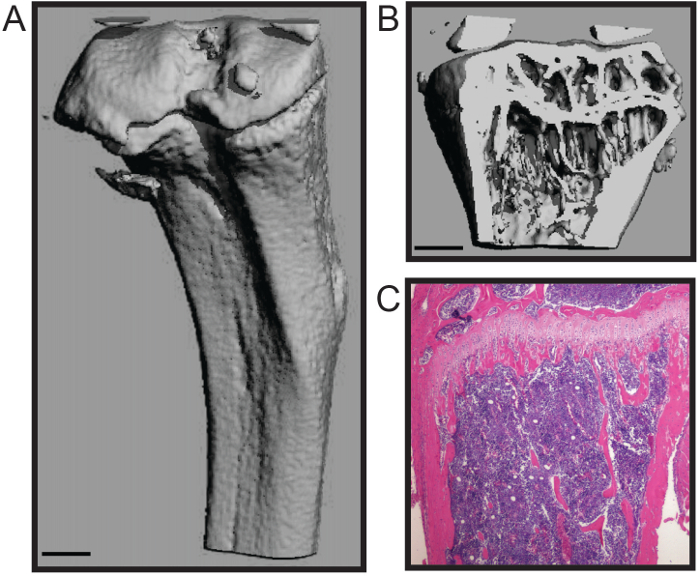
Reconstruction Figure 1. histomorphologique et analyses histologiques de souris os long. Trois dimensions microCT d'un tibia de souris montrant (A) la coque corticale externe et (B trong>) os trabéculaire (barre d'échelle = 0,5 mm). (C) histologiques H & E tache d'un tibia décalcifiée et en coupe (4x). Images courtoisie de Katherine Weilbaecher, School of Medicine, USA Université de Washington. S'il vous plaît cliquer ici pour voir une version plus grande de cette figure.

Figure 2. Primary Bone Marrow culture cellulaire pour la différenciation des ostéoclastes et des ostéoblastes. (A) TRAP coloration pour ostéoclastes multinucléés après 7 jours dans les médias ostéoclastogénique (4x). (B) La phosphatase alcaline (couleur violette) pour ostéoblastes et alizarine rouge (couleur rouge) tache pour la minéralisation après 21 jours dans les médias ostéogénique. Images courtoisie de Katherine Weilbaecher, School of Medicine, USA Université de Washington.iles / ftp_upload / 53936 / 53936fig2large.jpg "target =" _ blank "> S'il vous plaît cliquer ici pour voir une version plus grande de cette figure.
Discussion
We present a simple and efficient method for removal of mouse hind long bones and subsequent bone marrow isolation. This method maintains the high structural and cellular integrity of the bones and bone marrow and has low handling time, minimizing the likelihood of user-induced fracture or bone scoring that may influence downstream analyses. In addition, the centrifugation method for isolating bone marrow does not require cutting the bone to expose the bone marrow space or fluid to flush the bone marrow, reducing potential points of contamination. Moreover, the centrifuge technique is relatively high-throughput with lower hands-on time than other methods, thus reducing processing time.
High variation is inherent to in vivo mouse studies due to high mouse-to-mouse phenotypic variation. In order to maximize the research impact of expensive and labor-intensive mouse studies, it is critical to minimize technical experimental error9,10. Time from animal sacrifice to downstream analysis or tissue fixation introduces experimental variation that may overcome subtle changes and reduce large differences between groups. Therefore, rapid processing of samples is essential for accurate data analysis. The long bone dissection and bone marrow isolation techniques described here are optimized for rapid processing of animals and samples to reduce technical variation.
This protocol can be widely applied to many research fields, including investigation of the bone tissue itself or interrogation of the cells of the bone marrow. In addition, this straightforward approach to long bone dissection will enable researchers in related fields to directly interrogate bone contributions in order to expand our knowledge of bone marrow dysfunction in otherwise understudied pathologies.
Acknowledgements
This work was supported by NCI grant nos. U54CA143803, CA163124, CA093900, and CA143055 to K.J.P. The authors thank the current and past members of the Weilbaecher lab, especially Katherine Weilbaecher, Michelle Hurchla, and Hongju Deng, and members of the Brady Urological Institute, especially members of the Pienta laboratory for critical reading of the manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| pinboard | |||
| pins | |||
| 70% ethanol | |||
| dissection sissors | |||
| dissection forceps | |||
| Kimwipes | Kimberly-Clark | 34120 | |
| 16 guage needle | |||
| 1.5 ml microcentrifuge tube | |||
| 0.5 ml microcentrifuge tube | |||
| microcentrifuge |
References
- McHayleh, W. M., Ellerman, J., Roodman, G. D. Ch. 80. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. , 379-381 (2008).
- Weilbaecher, K. N., Guise, T. A., McCauley, L. K. Cancer to bone: a fatal attraction. Nat Rev Cancer. 11 (6), 411-425 (2011).
- Pedersen, E. A., Shiozawa, Y., Pienta, K. J., Taichman, R. S. The prostate cancer bone marrow niche: more than just 'fertile soil. Asian J Androl. 14 (3), 423-427 (2012).
- Amend, S. R., et al. Thrombospondin-1 regulates bone homeostasis through effects on bone matrix integrity and nitric oxide signaling in osteoclasts. J Bone Miner Res. 30 (1), 106-115 (2015).
- Hurchla, M. A., et al. The epoxyketone-based proteasome inhibitors carfilzomib and orally bioavailable oprozomib have anti-resorptive and bone-anabolic activity in addition to anti-myeloma effects. Leukemia. 27 (2), 430-440 (2013).
- Rauch, D. A., et al. The ARF tumor suppressor regulates bone remodeling and osteosarcoma development in mice. PLoS One. 5 (12), e15755 (2010).
- Su, X., et al. The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. J Clin Invest. 122 (10), 3579-3592 (2012).
- Dempster, D. W., et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 28 (1), 2-17 (2013).
- Begley, C. G., Ellis, L. M. Drug development: Raise standards for preclinical cancer research. Nature. 483 (7391), 531-533 (2012).
- Festing, M. F., Altman, D. G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 43 (4), 244-258 (2002).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved