Murin bakbenen rörben Dissection och benmärg Isolering
In This Article
Summary
Here we present a protocol for the dissection of hind limb long bones (femurs and tibiae) from the laboratory mouse. We further describe a rapid technique for bone marrow isolation from these bones that utilizes centrifugation for removal of bone marrow from the bone marrow space.
Abstract
Investigation of the bone and the bone marrow is critical in many research fields including basic bone biology, immunology, hematology, cancer metastasis, biomechanics, and stem cell biology. Despite the importance of the bone in healthy and pathologic states, however, it is a largely under-researched organ due to lack of specialized knowledge of bone dissection and bone marrow isolation. Mice are a common model organism to study effects on bone and bone marrow, necessitating a standardized and efficient method for long bone dissection and bone marrow isolation for processing of large experimental cohorts. We describe a straightforward dissection procedure for the removal of the femur and tibia that is suitable for downstream applications, including but not limited to histomorphologic analysis and strength testing. In addition, we outline a rapid procedure for isolation of bone marrow from the long bones via centrifugation with limited handling time, ideal for cell sorting, primary cell culture, or DNA, RNA, and protein extraction. The protocol is streamlined for rapid processing of samples to limit experimental error, and is standardized to minimize user-to-user variability.
Introduction
The study of long bones and the cells of the bone marrow is central to a myriad of research disciplines, including, but not limited to, bone biology, cancer biology, immunology, hematology, and biomechanics. The bone is a highly dynamic organ that together with the cartilage forms the skeleton to provide mechanical support against loading and protection of the internal organs. In addition, the mineral components of bone are a storage sink for the critical signaling molecules calcium and phosphorus, as well as other factors1. Finally, bones house the bone marrow and, together with metabolically active bone forming osteoblasts and bone resorbing osteoclasts, provide the stem cell niche necessary for the maintenance of hematopoietic and lymphoid cell populations.
Bone and bone marrow are affected in many disorders, often leading to bone marrow dysfunction, severe bone pain, and pathologic fracture. Bone is a common site of metastasis in many solid tumors, most notably breast cancer and prostate cancer, where tumor cells directly engage the bone marrow niche to initiate the vicious cycle of bone metastasis and displace hematopoietic stem cells2,3. Hematopoietic malignancies including myeloma and leukemia are characterized by bone marrow dysfunction as well as deregulation of healthy bone remodeling1. Other non-malignant skeletal disorders are also active areas of research, such as osteoarthritis, osteoporosis, scoliosis, and rickets. Even in an otherwise healthy individual, biomechanical failure in a bone leads to a painful fracture. All of these disorders represent active areas of research with the goal of identifying new preventative measures and treatment regimens to reduce morbidity and mortality.
To research the plethora of roles of the bone and the bone marrow, both under physiologic and pathologic conditions, it is critical for researchers to have a simple and efficient standardized method for dissection of the mouse long bones for rapid processing of large in vivo experiments. The dissection protocol outlined here is suitable for all long bone analyses including ex vivo imaging, histology, histomorphometry, and strength testing, among others. Similarly, a standardized bone marrow isolation method with high bone marrow cell recovery and low inter-user variability is important for experimental analysis such as fluorescence-activated cell sorting (FACS) or quantitative PCR (qPCR) as well as downstream applications such as primary cell culture of bone marrow cells.
Protocol
Alla djur arbete godkändes av Institutional Animal Care och användning kommittén i enlighet med rekommendationerna som beskrivs i handledningen för vård och användning av försöksdjur i National Institutes of Health.
1. bakbenen Long Bone Dissection
- Avliva musen i enlighet med institutionens riktlinjer.
- Placera musen i ryggläge och anbringa genom att klämma fast alla fyra benen genom de mus tassarna nedan fotleden.
- Spraya musen med 70% etanol, grundligt dousing benen.
- Gör ett litet snitt till höger om mittlinjen i nedre delen av buken, precis ovanför höften.
- Förlänga incisionen ner benet och förbi fotleden.
- Dra tillbaka huden och skär quadricepsmuskeln förankrad i proximala änden av lårbenet för att exponera den främre sidan av lårbenet och stift ut från benet, placera tappen på en 45-graders vinkel från brädet.
- Med blade på saxen mot den bakre sidan av lårbenet, skär hamstrings bort från knäleden.
- Dra tillbaka huden och hamstringsmusklerna förankrade till proximala änden av lårbenet för att exponera den bakre sidan av lårbenet och stift ut från benet, placera tappen på en 45-graders vinkel från brädet.
- Med pincett, håll den distala änden av lårbenet, precis ovanför knäleden. Guide bladen i saxen på vardera sidan av lårbensskaftet mot höftleden, försiktigt så att inte skära in i lårbenet själv.
- Efter att ha nått lårbenshuvudet, vilket indikeras av saxen öppnas något, vrid saxen med den övre bladet på saxen rör sig direkt över lårbenshuvudet för att flytta ut lårbenet, försiktigt så att inte knäppa benet under lårbenshuvudet.
- Ta tag i övre delen av lårbenet med pincett, skära mjuka vävnaden bort från lårbenshuvudet för att frigöra den från acetabulum.
- Dra hela benet ben, inklusivefemur, knä, och skenbenet, upp och bort från kroppen, att försiktigt skäras bort bindväv och muskler som förbinder benet till huden.
- Overextend fotleden och återigen använda saxen i en vridande rörelse för att flytta ut den skenbenet.
- Greppa den distala änden av skenbenet, till att inte avskilja senorna, dra skenbenet uppåt och bort från kroppen och stiftet kortet.
- Skär eventuellt kvarvarande bindväv fästa långt ben till musen vid knäet.
- Avlägsnande av eventuellt extra muskel eller bindväv fäst vid lårbenet och skenbenet.
- För alla program som kräver benet förbli intakt (histologi, histomorfometri, biomekanisk testning, osv.), Fortsätt med standard interna protokoll (som i 4-7). För att isolera benmärgen, gå vidare till avsnitt 2.
2. Långa ben Förberedelse för Benmärgs Isolering
- Med hjälp av pincett, ta tag i lårbenet med knäskålen vänd bort ennd den proximala änden (lårbenshuvudet) nedåt.
- Overextend knäleden och använda saxen i en vridande rörelse för att flytta ut skenbenet och lårbenet.
- Klipp någon bindväv håller lårben och skenben tillsammans.
- Med användning av pincett, ta tag i lårbenet med den främre sidan som är vänd bort och den proximala änden (lårbenshuvudänden) nedåt.
- Guide saxen upp lårbensskaftet till kondylerna.
- Rotera försiktigt saxen fram och tillbaka för att ta bort kondylerna, knäskålen och epifysen att exponera metafysen.
- Ta bort eventuella extra muskler eller bindväv fäst vid lårbenet med hjälp av pincett, sax och Kimwipes.
- Med hjälp av pincett, ta tag i skenbenet med den främre sidan vänd bort och den bortre änden (fotled slut) nedåt.
- Om skenbens epifysen är intakt, vägleda saxen upp tibia axeln till kondylerna.
- Rotera försiktigt saxen fram och tillbaka för att ta bort kondylerna och epifysen att exponera metaphlys.
- Ta bort eventuella extra muskler eller bindväv fäst vid skenbenet med pincett, sax och Kimwipes.
3. Benmärgs Isolering
- Knuffa en 18 G nål genom botten av ett 0,5 ml mikrocentrifugrör.
- Placera långa ben (max 2 lårben och två tibiae) i röret, knä-end ner slut ner och stäng locket.
- Kapsla 0,5 ml mikrocentrifugrör i en 1,5 ml mikrocentrifugrör.
- Centrifugera kapslade rören vid ≥10,000 xg i en mikrocentrifug under 15 sek.
- Verifiera att benmärgen har spunnits ut ur benen genom visuell inspektion. Benen ska visas vita och det bör finnas en stor visuell pellets i det grövre röret.
- Kasta 0,5 ml mikrocentrifugrör med benen.
- Suspendera benmärgen i lämplig lösning (t.ex. PBS, odlingsmedier, FACS-buffert) och fortsätter med försöksprotokoll (DNA, RNA eller proteinisolering,FACS-analys, eller primär cellkultur).
Representative Results
Protokollet som beskrivs här är optimerad för snabb dissekering av musen lårbenet och skenbenet med ett minimum av skada på benvävnad. Denna teknik är lämplig för ett antal analyser nedströms, inklusive biomekanik studier, histomorfometri (Figur 1A - B), och histologi (Figur 1C) 4,7. Den representativa histomophometric micoCT 3D-rekonstruktion (Figur 1A - B) visar att både den porösa benet och kortikala skal bibehålls som möjliggör noggrann kvantifiering av de standardiserade strukturella parametrar för benhistomorfometri, inklusive trabekulära antal, tjocklek och avstånd; benvolym; och kortikal tjocklek, bland andra åtgärder 8. Den representativa histologiska avsnitt visar en H & E färgade formalinfixerade och urkalkade tibia (Figur 1C). Bilden visar integritet både förkalkad ben och cellulär benmärg för histologisk analys.
Benmärgen isoleringsförfarandet bevarar steriliteten hos benmärgen utrymme, har låg hantering för att minska kontaminering, och som inte kräver skärning av den långa ben, vilket minskar förlusten av benmärgs utbyte. Denna benmärg är lämplig för många tillämpningar nedströms, inklusive flödescytometri 5 och PCR-analyser. Dessutom kan detta förfarande användas för att isolera benmärg för primär cellkultur av benmärgsceller, inklusive osteoklaster och osteoblaster (Figur 2A - B) 4,6.
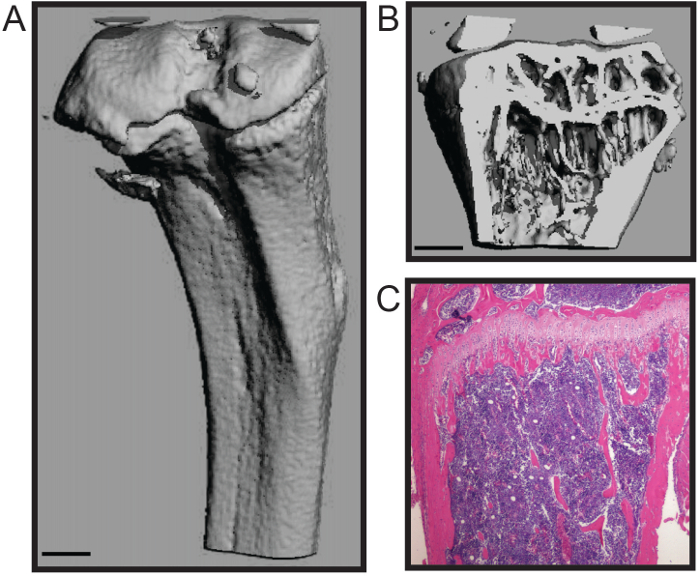
Figur 1. Histomorphological och histologiska analyser av mus rörben. Tredimensionell microCT rekonstruktion av en mus tibia visar (A) den yttre kortikala skalet och (B Trong>) trabekulärt ben (skala bar = 0,5 mm). (C) Histologisk H & E färgning av en avkalkat och sektionerad tibia (4x). Bilder med tillstånd av Katherine Weilbaecher, Washington University School of Medicine, USA. Klicka här för att se en större version av denna siffra.

Figur 2. Primär Bone Marrow Cell Culture för Differentiering av osteoklaster och osteoblaster. (A) TRAP-färgning för multinukleära osteoklaster efter 7 dagar i osteoclastogenic media (4x). (B) alkaliskt fosfatas (lila färg) för osteoblaster och alizarin röd (röd färg) fläck för mineralisering efter 21 dagar i osteogent media. Bilder artighet av Katherine Weilbaecher, Washington University School of Medicine, USA.iles / ftp_upload / 53936 / 53936fig2large.jpg "target =" _ blank "> Klicka här för att se en större version av denna siffra.
Discussion
We present a simple and efficient method for removal of mouse hind long bones and subsequent bone marrow isolation. This method maintains the high structural and cellular integrity of the bones and bone marrow and has low handling time, minimizing the likelihood of user-induced fracture or bone scoring that may influence downstream analyses. In addition, the centrifugation method for isolating bone marrow does not require cutting the bone to expose the bone marrow space or fluid to flush the bone marrow, reducing potential points of contamination. Moreover, the centrifuge technique is relatively high-throughput with lower hands-on time than other methods, thus reducing processing time.
High variation is inherent to in vivo mouse studies due to high mouse-to-mouse phenotypic variation. In order to maximize the research impact of expensive and labor-intensive mouse studies, it is critical to minimize technical experimental error9,10. Time from animal sacrifice to downstream analysis or tissue fixation introduces experimental variation that may overcome subtle changes and reduce large differences between groups. Therefore, rapid processing of samples is essential for accurate data analysis. The long bone dissection and bone marrow isolation techniques described here are optimized for rapid processing of animals and samples to reduce technical variation.
This protocol can be widely applied to many research fields, including investigation of the bone tissue itself or interrogation of the cells of the bone marrow. In addition, this straightforward approach to long bone dissection will enable researchers in related fields to directly interrogate bone contributions in order to expand our knowledge of bone marrow dysfunction in otherwise understudied pathologies.
Acknowledgements
This work was supported by NCI grant nos. U54CA143803, CA163124, CA093900, and CA143055 to K.J.P. The authors thank the current and past members of the Weilbaecher lab, especially Katherine Weilbaecher, Michelle Hurchla, and Hongju Deng, and members of the Brady Urological Institute, especially members of the Pienta laboratory for critical reading of the manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| pinboard | |||
| pins | |||
| 70% ethanol | |||
| dissection sissors | |||
| dissection forceps | |||
| Kimwipes | Kimberly-Clark | 34120 | |
| 16 guage needle | |||
| 1.5 ml microcentrifuge tube | |||
| 0.5 ml microcentrifuge tube | |||
| microcentrifuge |
References
- McHayleh, W. M., Ellerman, J., Roodman, G. D. Ch. 80. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. , 379-381 (2008).
- Weilbaecher, K. N., Guise, T. A., McCauley, L. K. Cancer to bone: a fatal attraction. Nat Rev Cancer. 11 (6), 411-425 (2011).
- Pedersen, E. A., Shiozawa, Y., Pienta, K. J., Taichman, R. S. The prostate cancer bone marrow niche: more than just 'fertile soil. Asian J Androl. 14 (3), 423-427 (2012).
- Amend, S. R., et al. Thrombospondin-1 regulates bone homeostasis through effects on bone matrix integrity and nitric oxide signaling in osteoclasts. J Bone Miner Res. 30 (1), 106-115 (2015).
- Hurchla, M. A., et al. The epoxyketone-based proteasome inhibitors carfilzomib and orally bioavailable oprozomib have anti-resorptive and bone-anabolic activity in addition to anti-myeloma effects. Leukemia. 27 (2), 430-440 (2013).
- Rauch, D. A., et al. The ARF tumor suppressor regulates bone remodeling and osteosarcoma development in mice. PLoS One. 5 (12), e15755 (2010).
- Su, X., et al. The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. J Clin Invest. 122 (10), 3579-3592 (2012).
- Dempster, D. W., et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 28 (1), 2-17 (2013).
- Begley, C. G., Ellis, L. M. Drug development: Raise standards for preclinical cancer research. Nature. 483 (7391), 531-533 (2012).
- Festing, M. F., Altman, D. G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 43 (4), 244-258 (2002).
Explore More Articles
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved