A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Dissection and Observation of Honey Bee Dorsal Vessel for Studies of Cardiac Function
In This Article
Summary
The abdominal dorsal vessel of the honey bee and other insects serves as the functional equivalent of the mammalian heart and plays an important role in nutrient transport, waste removal, immune function, and more. Here we describe a protocol for the visualization and pharmacological manipulation of bee heart rate.
Abstract
The European honey bee, Apis mellifera L., is a valuable agricultural and commercial resource noted for producing honey and providing crop pollination services, as well as an important model social insect used to study memory and learning, aging, and more. Here we describe a detailed protocol for the dissection of the dorsal abdominal wall of a bee in order to visualize its dorsal vessel, which serves the role of the heart in the insect. A successful dissection will expose a functional heart that, under the proper conditions, can maintain a steady heartbeat for an extended period of time. This allows the investigator to manipulate heart rate through the application of cardiomodulatory compounds to the dorsal vessel. By using either a digital microscope or a microscope equipped with a digital camera, the investigator can make video recordings of the dorsal vessel before and after treatment with test compounds. The videos can then be scored at a time convenient to the user in order to determine changes in heart rate, as well as changes in the pattern of heartbeats, following treatment. The advantages of this protocol are that it is relatively inexpensive to set up, easy to learn, requires little space or equipment, and takes very little time to conduct.
Introduction
The overall goal of this methodology is to allow the investigator to quickly and easily observe and quantify the effect that a pharmacological agent has on the heart rate of honey bees. Bees, like other insects, have an open circulatory system that disseminates hemolymph, the insect equivalent of blood, throughout the body cavity, known as the hemocoel. The circulation of hemolymph is essential for the transport of nutrients, immune factors, waste products, as well neurohormones and other signaling molecules1. Circulation is facilitated by the dorsal vessel, which extends along the dorsal midline of the insect, as well as accessory pulsatile organs. The dorsal vessel is divided into two functionally distinct sections, designated the heart in the abdomen and the aorta in the thorax and head. Propagated contractions in the heart pump hemolymph towards the thorax and head, while accessory pulsatile organs ensure hemolymph flow to the extremities.
Insect cardiac function can be observed using a variety of methods, depending upon the size, physiology, or life stage of the insect. A common approach for observing heart rate in larvae or smaller insects is the use of intravital imaging2. This method is less useful in adult bees, however, as it can be difficult to clearly view the dorsal vessel through the abdominal wall. An established approach for recording heart rate in a variety of insects, including bees, is the use of contact thermography, which utilizes thermistors applied to the exterior of the insect to detect cardiac pulsations3,4. Heart rate in adult bees has also been recorded using an electrophysiological technique to measure an electrical impedance signal4,5. This technique requires the insertion of electrodes into the animal next to the heart and the use of an impedance converter to record heartbeats4. Similarly, electrocardiograms have been used to detect electrical signals produced by the heart and combined with video recording of the bee to observe changes in cardiac activity6. A distinct advantage to these approaches is that heart rate is assessed in an intact, living bee, rather than in a dissected specimen, which helps to ensure the availability of the full range of physiological responses in the subject. The challenges of these approaches include accounting for immobilization or anesthetization of the subject, the need to limit outside variables and stimuli that might alter heart rate, as well as determining an appropriate delivery method when testing pharmacological agents.
Another approach that has been used for studying bee cardiac activity is to partially dissect the insect in order to expose the heart, then measure dorsal vessel contractions using a force displacement transducer7. In this protocol, the heart is continually bathed with running physiological saline and test compounds can be dissolved in this solution for application to the subject7. A significant difference between this method and those previously described is that the ventral nerve cord is removed, eliminating the role that the central nervous system has been shown to play in modulating heart rate5. The result is that the baseline heartbeat, which is usually quite erratic, stabilizes at a much lower frequency and amplitude than is typically observed in a living insect5,7. What all of these methods have in common is that they require highly specialized and often expensive equipment, in addition to a certain level of expertise, in order to be conducted. Perhaps the greatest disadvantage is that none of these approaches are particularly well suited to experiments that involve testing a large number of subjects, such as screening a library of potentially cardiomodulatory compounds.
The greatest strength of the approach described here is its simplicity. The protocol is relatively easy to master, the setup requires little space, and only a minimal financial input is necessary. The method requires little more than some bees, a few surgical instruments, an isotonic solution, and either a digital microscope or a traditional microscope with a digital camera. Bees are dissected to visualize the dorsal vessel and digital videos are used to record heart rate before and after treatment with pharmacological agents. Although video recording is not actually necessary to observe changes in heart rate, it will greatly increase throughput (i.e., the number of subjects that can be processed in a given amount of time). The investigator can maximize efficiency by recording a large number of videos at once and then later scoring these videos at a more convenient time. Another advantage of this approach is that videos allow the investigator to start over, should the scoring process be interrupted, and make it easier for the viewer to be blinded to the treatment in order to reduce bias.
Protocol
1. Collection and Preparation of Test Subjects
- Collect the appropriate number of bees from the colony.
NOTE: The number needed depends upon not only the size and scope of the experiment, but also the skill of the investigator. For example, if there are 2 treatment groups with a desired sample size of 10 bees per group, a reasonably skilled investigator might collect at least 30 bees to account for unsuccessful dissections and end up with 20 useful videos to score. - Minimize the amount of time that passes between collection and dissection.
NOTE: Although bees can be housed in the lab for days prior to dissection, the success rate of dissections (i.e., the likelihood of maintaining a stable heart rate in a dissected dorsal vessel) has been observed to decrease relative to the amount of time that bees are housed away from the colony.- Provide bees with a source of water and food while housed in the lab. For example, at a minimum, provide access to a 50% (w/v) solution of sucrose in water (this is sufficient for durations of less than 6 hr). For longer periods, provide bees access to honey.
- House bees in the lab overnight at a temperature of approximately 32 °C and 60-80% relative humidity to reduce stress and avoid dehydration.
- Prior to dissection, anesthetize bees briefly to aid in handling.
NOTE: This can decrease the success rate of dissections and reduce throughput.- Chill the bees either by placing them on ice or into a refrigerator for just long enough to reduce movement in order to aid in handling.
- Alternatively, briefly expose bees to CO2 in order to aid in handling.
NOTE: Extended exposure to cold can reduce the success rate of dissections. Extended or repeated exposure to CO2 can also reduce the success rate of dissections.
2. Dissection of Dorsal Abdominal Wall
NOTE: Bees should be alive at the time of dissection.
- Using forceps and/or microdissection scissors, remove legs and wings to facilitate dissection of the abdomen. Keep a small beaker or similar container filled with distilled water nearby for the purpose of rinsing instruments between dissections.
- While restraining the bee with forceps, utilize the microdissection scissors to cut laterally along the dorsal abdominal wall between the first and second tergites (see Figure 1).
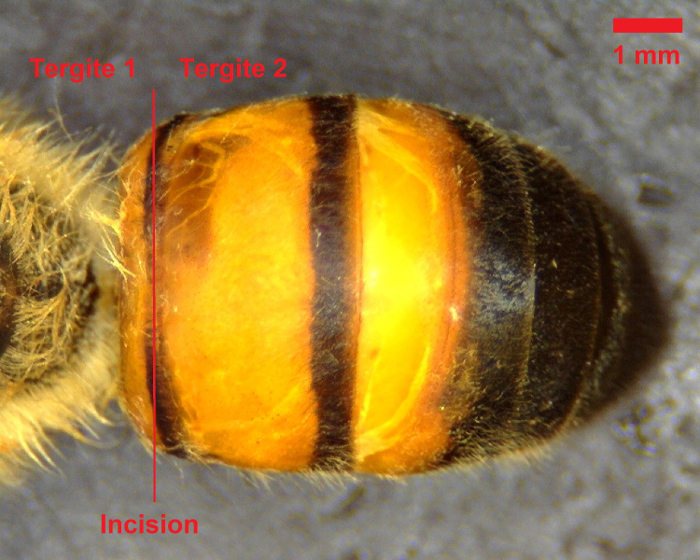
Figure 1: Dorsal view of bee abdomen. The initial incision should be made between the first and second tergites, as denoted by the red line. Scale bar = 1 mm. Please click here to view a larger version of this figure.
- While lightly gripping the posterior edge of the second tergite with the forceps, cut longitudinally along each side of the bee from the initial incision to the stinger (see Figure 2). Use caution when cutting to avoid puncturing the gastrointestinal tract.

Figure 2: Lateral view of bee abdomen. The second and third incisions should be made along either side of the abdomen, as denoted by the red line. Scale bar = 1 mm. Please click here to view a larger version of this figure.
- Exchange the scissors for a second set of fine forceps and utilize them to carefully separate the dorsal abdominal wall from the rest of the abdomen. Gently remove the stinger and any portion of the gastrointestinal tract that remains attached to the dorsal abdominal wall. Avoid rupturing the gut, as the contents can coat the abdominal wall and impede visualization of the dorsal vessel.

Figure 3: View of the dorsal vessel. Once the gut and stinger have been removed, the dorsal vessel is visible along the midline of the dissected dorsal abdominal wall. Scale bar = 1 mm. Please click here to view a larger version of this figure.
- Arrange the dorsal abdominal wall in the desired orientation beneath the camera so that the dorsal vessel is visible (see Figure 3). Utilize the microdissection scissors to trim away any excess abdominal wall that impedes visualization of the dorsal vessel. The shape of the dorsal abdominal wall should resemble a shallow cup or bowl when properly situated.
- Since the dorsal vessel does not extend into the hindmost abdominal segment of the bee, remove the final tergite in order to improve visualization of the dorsal vessel.
- Utilizing an adjustable volume micropipette, cover the dorsal vessel with 10 µl of an isotonic solution to maintain physiological conditions and facilitate a steady heartbeat.
NOTE: The recommended solution is quarter strength Ringer's solution (0.120 g/L calcium chloride, 0.105 g/L potassium chloride, 0.050 g/L sodium bicarbonate, and 2.250 g/L sodium chloride), which has been found to facilitate a stable, continuous heartbeat.
3. Observation and Modulation of Heart Rate
- Allow the dorsal vessel to sit undisturbed until a stable, continuous heartbeat is achieved (usually within 300 sec).
NOTE: Heartbeat is visualized as rhythmic contractions of the dorsal vessel. Initially, there may appear to be no heartbeat, especially if the bee was anesthetized, but the heart will usually resume beating after resting in isotonic solution for several minutes and can continue beating for hours, provided it remains bathed in solution. - Measure the heart rate in terms of the number of beats per min (BPM).
- Record the number of contractions observed during a 60 sec period. Use a hand tally counter and a timer to facilitate this process.
- Measure the change in heart rate by recording the observed BPM before and after treatment with a cardiomodulatory compound.
NOTE: Although the time required to observe an effect on heart rate may vary depending upon the compound being tested, changes in heart rate can typically be observed within minutes.- Determine the baseline heart rate immediately prior to the addition of any test compound.
NOTE: Post-treatment heart rate can usually be determined after 90 to 120 sec. - Prepare potential cardiomodulators (e.g., octopamine) by dissolving the compound in the same isotonic solution used to bathe the dorsal vessel.
- Add the test compounds to the solution surrounding the dorsal vessel by utilizing a micropipetter.
- Determine the baseline heart rate immediately prior to the addition of any test compound.
- For greater accuracy and higher throughput, make a video recording of each test subject and then use the videos to score heart rate at a later time.
NOTE: This allows a single investigator to stagger dissections in order to facilitate almost continuous production of videos. When recording videos, the minimum recommended length is approximately 240 sec with any test compound being added at the 60 sec mark. This ensures that the investigator has a 60 sec window for scoring baseline heart rate and then another 60 sec window for scoring post-treatment heart rate 120 sec after treatment.
Results
Since many of the pharmacologically active compounds that might be tested using this protocol are not soluble in water, it is necessary to have a reliable solvent that will allow test compounds to be delivered via the isotonic solution used to bathe the dorsal vessel. Dimethyl sulfoxide (DMSO) is a solvent that is commonly used as a vehicle for delivering experimental drugs and other compounds in animals8, and it has been used successfully for this purpose in studies examining ...
Discussion
The protocol presented here provides a simple and effective approach to testing pharmacological compounds for their effects on honey bee heart rate. As observed in prior experiments that either transect the ventral nerve cord of a living insect5 or dissect out the ventral nerve cord when exposing the dorsal vessel7, the loss of central nervous system regulation results in a stable, low frequency heartbeat. The low frequency of beats allows the investigator to visually assess heart rate without havin...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Drs. Jeffrey Bloomquist and Daniel Swale for their technical comments and suggestions. This project was partially funded by the Department of Entomology and the College of Agriculture and Life Sciences at Virginia Tech.
Materials
| Name | Company | Catalog Number | Comments |
| Dino-Lite Edge digital USB microscope | Dino-Lite | AM4815ZT | Any digital microscope or similar setup will suffice |
| Microscope stand | Dino-Lite | RK-10 | Any stand appropriate for the digital microscope |
| Laptop or PC | Necessary for digital microscope | ||
| Microdissection scissors (Vannas, 8 cm, Straight, 5 mm Blades) | World Precision Instruments | 14003 | Any similar scissors suitable for microdissection will suffice |
| Microdissecting Forceps, 10.2 cm, Angled (2 pair) | World Precision Instruments | 504482 | Any similar forceps suitable for microdissection will suffice |
| Ringers solution 1/4 strength tablets | Sigma-Aldrich | 96724-100TAB | |
| Dissecting tray | Any surface suitable for microdissection | ||
| Single channel 10 µl pipette | Any device capable of accurately delivering 10 µl volume | ||
| Pipette tips | |||
| Small beaker or container of water | Used to rinse instruments between subjects | ||
| Hand tally counter | Office Depot | 295033 | Any similar product will suffice |
| Timer | Office Depot | 644219 | Any similar product will suffice |
| Deionized water | Preparation of Ringers solution and rinsing instruments |
References
- Klowden, M. J. Circulatory Systems. Physiological Systems in Insects, 3rd Edition. , 365-413 (2013).
- League, G. P., Onuh, O. C., Hillyer, J. F. Comparative structural and functional analysis of the larval and adult dorsal vessel and its role in hemolymph circulation in the mosquito Anopheles gambiae. J Exp Biol. 218 (Pt 3), 370-380 (2015).
- Wasserthal, L. T. Oscillating Hemolymph Circulation in the Butterfly Papilio-Machaon L Revealed by Contact Thermography and Photocell Measurements. J Comp Physiol. 139 (2), 145-163 (1980).
- Wasserthal, L. T. Interaction of circulation and tracheal ventilation in holometabolous insects. Adv Insect Physiol. 26, 297-351 (1996).
- Schwab, E. R., Chilson, R. A., Eddleman, C. D. Heartbeat Rate Modulation Mediated by the Ventral Nerve Cord in the Honey-Bee, Apis-Mellifera. J Comp Physiol B-Biochem Syst Environ Physiol. 161 (6), 602-610 (1991).
- Kaiser, W., Weber, T., Otto, D., Miroschnikow, A. Oxygen supply of the heart and electrocardiogram potentials with reversed polarity in sleeping and resting honey bees. Apidologie. 45 (1), 73-87 (2014).
- Papaefthimiou, C., Theophilidis, G. Octopamine--a single modulator with double action on the heart of two insect species (Apis mellifera macedonica and Bactrocera oleae): Acceleration vs. inhibition. J Insect Physiol. 57 (2), 316-325 (2011).
- Castro, C. A., Hogan, J. B., Benson, K. A., Shehata, C. W., Landauer, M. R. Behavioral-Effects of Vehicles - Dmso, Ethanol, Tween-20, Tween-80, and Emulphor-620. Pharmacol Biochem Behav. 50 (4), 521-526 (1995).
- Papaefthimiou, C., Papachristoforou, A., Theophilidis, G. Biphasic responses of the honeybee heart to nanomolar concentrations of amitraz. Pestic Biochem Phys. 107 (1), 132-137 (2013).
- Roeder, T. Octopamine in invertebrates. Prog Neurobiol. 59 (5), 533-561 (1999).
- Johnson, E., Ringo, J., Dowse, H. Modulation of Drosophila heartbeat by neurotransmitters. J Comp Physiol B. 167 (2), 89-97 (1997).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved