A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Preparation and Evaluation of 99mTc-labeled Tridentate Chelates for Pre-targeting Using Bioorthogonal Chemistry
* These authors contributed equally
In This Article
Summary
Here, we describe a protocol for radiolabeling and in vivo testing of tridentate 99mTc(I) chelate-tetrazine derivatives for pre-targeting and bioorthogonal chemistry.
Abstract
Pre-targeting combined with bioorthogonal chemistry is emerging as an effective way to create new radiopharmaceuticals. Of the methods available, the inverse electron demand Diels-Alder (IEDDA) cycloaddition between a radiolabeled tetrazines and trans-cyclooctene (TCO) linked to a biomolecule has proven to be a highly effective bioorthogonal approach to imaging specific biological targets. Despite the fact that technetium-99m remains the most widely used isotope in diagnostic nuclear medicine, there is a scarcity of methods for preparing 99mTc-labeled tetrazines. Herein we report the preparation of a family of tridentate-chelate-tetrazine derivatives and their Tc(I) complexes. These hitherto unknown compounds were radiolabeled with 99mTc using a microwave-assisted method in 31% to 83% radiochemical yield. The products are stable in saline and PBS and react rapidly with TCO derivatives in vitro. Their in vivo pre-targeting abilities were demonstrated using a TCO-bisphosphonate (TCO-BP) derivative that localizes to regions of active bone metabolism or injury. In murine studies, the 99mTc-tetrazines showed high activity concentrations in knees and shoulder joints, which was not observed when experiments were performed in the absence of TCO-BP. The overall uptake in non-target organs and pharmacokinetics varied greatly depending on the nature of the linker and polarity of the chelate.
Introduction
99mTc remains the dominant radioisotope used in diagnostic nuclear medicine, with over 50 million imaging procedures conducted per year worldwide1,2,3. The majority of 99mTc agents used clinically are perfusion type radiopharmaceuticals. There are a limited number of actively targeted compounds in which 99mTc is directed to bind a specific biomarker through ligation to a targeting construct. The creation of targeted 99mTc radiopharmaceuticals is often hindered by the influence of 99mTc-ligand complexes on the ability of the targeting molecule to bind the biomarker of interest, or the isotopes half-life is not long enough for use with higher molecular weight biomolecules such as antibodies. The latter typically requires several days before images are acquired in order for the biomolecule to clear from non-target tissues. Pre-targeting offers an alternative approach to overcome these challenges.
Pre-targeting combined with bioorthogonal chemistry has been shown to be an effective way to develop new molecular imaging probes for both fluorescence and radio-imaging4,5,6,7,8. The inverse electron demand Diels-alder (IEDDA) reaction between 1,2,4,5-tetrazine (Tz) and trans-cyclooctene (TCO) derivatives, as shown in Figure 1, has been shown to be particularly effective6. The IEDDA reaction with these components can exhibit fast kinetics in PBS (k2 ≈ 6,000 M-1 s-1) and high selectivity, making it ideal for in vivo pre-targeting applications9,10.
The most common approach used involves administering a TCO-derived targeting vector and following a sufficient delay period, a radiolabeled tetrazine is administered. Radiolabeled tetrazines based on 11C, 18F, 64Cu, 89Zr, and 111In have been reported11,12,13,14,15. In contrast, there is only one report of a 99mTc-labeled Tz, which was prepared using a HYNIC type ligand requiring the use of co-ligands to prevent protein binding and degradation in vivo16. As an alternative, we report here the synthesis of 99mTc(I) labeled tetrazines using a family of ligands which form stable tridentate complexes with a [99mTc(CO)3]+ core.
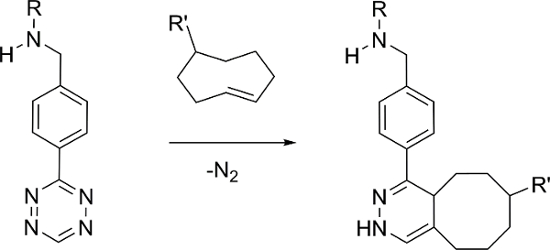
Figure 1: The bioorthogonal IEDDA reaction between tetrazine and trans-cyclooctene. Please click here to view a larger version of this figure.
The family of ligands prepared contain tridentate chelates that vary in polarity and the nature of the linker group between the metal binding region and the Tz (Figure 2). The goal was to identify a 99mTc-Tetrazine construct that could effectively localize and react with TCO-labeled sites in vivo and rapidly clear when not bound, in order to yield high target-to-non-target ratios. To test the ligands, a TCO-derivative of a bisphosphonate (TCO-BP) was used17. We have shown previously that TCO-BP localizes to areas of active bone metabolism and can react with radiolabeled tetrazines in vivo18. It is a convenient reagent to test new tetrazines, because it can be prepared in a single step and experiments can be performed in normal mice where localization occurs primarily in the joints (knees and shoulders).
Protocol
Animal studies were approved by the Animal Research Ethics Board at McMaster University in accordance with Canadian Council on Animal Care (CCAC) guidelines.
1. Radiolabeling of Tz-tridentate Ligands with 99mTc
CAUTION: The following procedures require the use of radioactive compounds. Work should only be done in a licensed laboratory with adherence to safety and disposal regulations. Microwave reactions should be performed in a microwave specifically designed for chemical synthesis.
- Synthesis of [99mTc(CO)3(H2O)3]+ 19,20
- In a microwave vial, combine 8 mg K2[BH3CO2], 15 mg Na2CO3, 20 mg Na2B4O7·10H2O, and 25 mg KOCO[CH(OH)]2COONa·4H2O. Purge the vial for 10 min with argon gas.
- Add 4 mL of 99mTcO4- (~1,100 MBq, ~30 mCi) in 0.9% saline to the vial.
- Heat the reaction in a microwave for 3.5 min at 110 °C after 10 s of stirring to ensure thorough mixing of reagents.
- Adjust the pH of the solution to 3.5-4 using ~400 μL of 1 M HCl. Verify using pH paper.
- Radiolabeling of Tetrazine ligands 1-5
- Dissolve 2 mg of each ligand (compounds 1-5) in 250 μL MeOH21.
- Add 250 μL of [99mTc(CO)3(H2O)3]+ (~74 MBq, ~2 mCi) to each solution.
- Heat the reaction mixture using a microwave for 20 min at 60 °C.
NOTE: This step was identical for all 5 tetrazines. - For compounds 2- 5, evaporate the solvent and re-dissolve the resulting products in 1 mL of 1:1 v/v DCM:TFA.
- Heat the dissolved reaction products (2-5) at 60 °C in a microwave for 6 min (2-4) or 10 min (5).
- After cooling to room temperature, evaporate the solvent using an evaporator (36 °C, 8 mbar, 3 min, 6,000 rpm) and dissolve the dried compound in 1:1 ACN:H2O or 1:1 MeOH:H2O, prior to HPLC purification.
- Purify the 99mTc-labeled compounds (1-5), including separating the labeled product from unlabeled tetrazine ligand, using HPLC (C18 reversed-phase). Typically, use an elution gradient of 30:70 ACN:H2O (both with 0.1% TFA) to 40:60 ACN:H2O over 20 min (18 min) and a C18 analytical 4.6 x 100 mm column. Use both UV (254 nm) and gamma detection.
- Take a small sample of each labeled product and compare its HPLC retention time to that of a co-injected, non-radioactive, Re-labeled standard (0.125 mg in 20% methanol-H2O). The Re-labeled standard is identified in the UV HPLC trace, and will elute at the same time as the 99mTc-labeled compound in the γ-HPLC trace. This co-injection shows peaks at comparable retention times, confirming the identity of the 99mTc-labeled compound.
- Evaporate the solvent from HPLC fractions using an evaporator (36 °C, 8 mbar, 3 min, 6,000 rpm).
- Formulate the purified compound at a concentration of 7.4 kBq/µL in PBS, containing 0.5% BSA and 0.01% Tween-80.
- To ensure the labelled compounds are stable, perform an in vitro stability study. Incubate the formulated compound at 37 °C for 1, 4 and 6 h, injecting a small amount (3.7 MBq) of the mixture on the HPLC at each time point to assess stability.
2. Pre-targeted Bio-distribution Studies
- Preparation of animals
- Using 7-9 week old, female Balb/c mice (n=3), administer TCO-BP formulated in saline (20 mg/kg) (5 μg/μL), via tail-vein injection.
- Place mouse in physical restraint device, and identify the veins located on the lateral surfaces of the tail and wipe with an alcohol swab. At approximately 2 cm from the end of the tail, insert a 30-gauge needle at a shallow angle, parallel to the vein. Slowly depress the plunger to inject, remove needle and apply clean gauze sponge at injection site with slight pressure until bleeding stops.
- At 1 h post injection of TCO-BP, administer ~0.74 MBq (20 μCi) of 99mTc-tetrazine formulated in 100 μL of 0.5% BSA, 0.01% Tween-80 in PBS, via tail-vein injection.
- Bio-distribution studies
- At the desired time point (t = 6 h), anaesthetize the mice using 3% isoflurane and 2% oxygen gas mixture. Demonstrate a toe pinch withdrawal on the anesthetized mouse to ensure they are under surgical plane of anesthesia.
- Collect blood (1 mL) via cardiac puncture using a syringe pre-treated with heparin. Place mouse on its back with nose in the nose cone for continued anesthesia and locate the xiphoid process on the animal.
- Insert a 25 G needle, slightly to the left of the animal's midline under the xiphoid process, at a 20° angle. Fully insert the needle, and slowly pull back on the plunger to see blood in the needle hub if the heart was punctured. Slightly readjust the needle while holding the plunger if necessary, to puncture the heart. Slowly draw blood into the syringe.
- Euthanize the animal by cervical dislocation, while under anesthesia.
- Place each animal in a plastic bag and use a dose calibrator (99mTc setting) to measure the whole body activity level.
- Collect the following tissues and fluids in pre-weighed counting tubes: blood, bone (knee and shoulder), gall bladder, kidneys, liver, stomach (with contents), small intestines (with contents), large intestines and caecum (with contents), thyroid and trachea, urinary bladder with urine, and tail.
- Rinse appropriate tissues (excluding blood, gall bladder, and urinary bladder) in PBS to remove blood and blot dry before placing the tissues in appropriate counting tubes.
- Place animal carcass in a plastic bag and measure residual whole body activity using a dose calibrator.
- Weigh each tube containing a tissue sample. Subtract initial weight of the tube to obtain mass of the tissue.
- Use a dose calibrator (99mTc setting) to measure the amount of activity in a test sample (100 µL) at the time of injection for each mouse.
NOTE: This test sample is equal to the injection volume, thus giving the activity count at the time of injection. - At the time of tissue measurement, aliquot 5 µL of the test sample used previously. Use a multi-detector gamma counter (99mTc setting) and count to obtain the count per minute (CPM) for the 5 µL test sample.
- Use the two values obtained in 2.2.9 and 2.2.10 to calculate the activity and CPM relationship using equation 1 to obtain a conversion factor (CPM µCi-1).
(1)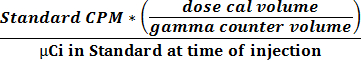
- Use the gamma counter to measure the amount of radioactivity in each tissue or fluid sample.
- Use equation 1 to calculate the amount of activity in each tissue or fluid at the time of measurement relative to the total injected dose. This value is then normalized by organ weight and reported as percent injected dose per gram (i.e., %ID/g) of tissue.
- Follow steps 2.1.2 to 2.2.13 to conduct a negative control experiment using the 99mTc-labeled tetrazine ligands in the absence of TCO-BP. Sacrifice mice (n = 3) at 0.5, 1, 4 and 6 h post injection and obtain tissue or fluid as described above.
Results
The ligands were synthesized using different linkers and chelators via a simple reductive amination strategy (Figure 2), followed by coupling of the product to a commercially available tetrazine22,23. Radiolabeling was performed using the same method for all compounds and was highly reproducible. The process was optimized by varying the pH, amount of ligand, reaction time and temperature whereupon the 99m
Discussion
A collection of tetrazine-linked tridentate chelates of varying polarities was prepared, and the utility of their 99mTc complexes in the IEDDA reaction with a TCO derivative in vivo was assessed. An effective and reproducible 99mTc labeling method was developed for five tetrazine-chelates, where the ligand concentration was 10-3 M. The labeling step was followed by deprotection of t-butyl groups (for compounds 2-5). The high concentration of ligand was u...
Disclosures
The authors declare they have no competing financial interests.
Acknowledgements
This work supported by research grant funding from the Natural Sciences and Engineering Research Council (NSERC) of Canada, the Ontario Institute for Cancer Research (OICR, #P.SI.015.8), and the Canadian Cancer Society (CCS, #703857). The authors acknowledge the contributions of Dr. Denis Snider who provided assistance in preparing the manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| Argon gas | Alphagaz | --- | --- |
| Na2CO3 | EMD Millipore | 106395 | --- |
| Na2B4O7·10H2O | Anachemia | S9640 | --- |
| KNaC4H4O6·4H2O | Anachemia | 217255 | --- |
| Technelite 99mTc generator | Lantheus medical imaging | --- | Source of 99mTcO4- |
| 0.9% Saline | Lantheus medical imaging | --- | To elute generator |
| 1 M HCl | Lab Chem | --- | --- |
| MeOH | Caledon | --- | --- |
| ACN | Caledon | --- | HPLC grade |
| Millipore H2O | Thermo Fisher Scientific | Barnstead Nanopure | --- |
| DCM | Caledon | --- | --- |
| TFA | Caledon | --- | --- |
| PBS | Thermo Fisher Scientific | 10010023 | pH 7.4 1x |
| BSA | Sigma Aldrich | A7906 | --- |
| Tween80 | Sigma Aldrich | P8047 | --- |
| Isoflurane | CDMV | 108737 | Supplier: Fresenius Kabi Animal Health |
| HPLC | Waters | 1525 Binary Pump, 2998 Photodiodde Array Detector, E-SAT/IN, Bioscan Flowcount PMT detector (item # 15590) | --- |
| HPLC column for analysis and purification of compounds 2-4 | Phenomenex | 00G-4435-E0 | Gemini® 5 µm C18 110 Å, LC Column 250 x 4.6 mm |
| HPLC column for analysis and purification of compounds 1 and 5 | Waters | 186003115 | XBridge BEH C18 Column, 130 Å, 5 µm, 4.6 mm x 100 mm |
| Microwave Reactor | Biotage | Initiator 8 | --- |
| Biotage V10 Evaporator | Biotage | Serial # V1041 | --- |
| Dose calibrator | Capintec, Inc. | CRC-25R | --- |
| Gamma counter | Perkin Elmer | Wizard 1470 Automatic Gamma Counter | --- |
| Animal room scale | Mettler Toledo | XP105 Delta Range | --- |
| Microwave vials | Biotage | 355629 | 0.5-2 mL |
References
- Jurisson, S. S., Lydon, J. D. Potential Technetium Small Molecule Radiopharmaceuticals. Chem. Rev. 99 (9), 2205-2218 (1999).
- Kluba, C. A., Mindt, T. L. Click-to-chelate: Development of Technetium and Rhenium-Tricarbonyl Labeled Radiopharmaceuticals. Molecules. 18, 3206-3226 (2013).
- Amato, I. Nuclear Medicines Conundrum. Chem. Eng. News. 87 (36), 58-70 (2009).
- Hnatowich, D. J., Virzi, F., Rusckowski, M. Investigations of Avidin and Biotin for Imaging Applications. J. Nucl. Med. 28 (8), 1294-1302 (1987).
- Blackman, M. L., Royzen, M., Fox, J. M. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels-Alder Reactivity. J. Am. Chem. Soc. 130 (41), 13518-13519 (2008).
- Devaraj, N. K., Weissleder, R., Hilderbrand, S. A. Tetrazine-Based Cycloadditions: Application to Pretargeted Live Cell Imaging. Bioconjugate Chem. 19 (12), 2297-2299 (2008).
- Rossin, R., et al. In Vivo Chemistry for Pretargeted Tumor Imaging in Live Mice. Angew. Chem., Int. Ed. 49 (19), 3375-3378 (2010).
- Zeglis, B. M., et al. Optimization of a Pretargeted Strategy for the PET Imaging of Colorectal Carcinoma via the Modulation of Radioligand Pharmacokinetics. Mol. Pharmaceutics. 12 (10), 3575-3587 (2015).
- Rossin, R., et al. Highly Reactive trans-Cyclooctene Tags with Improved Stability for Diels-Alder Chemistry in Living Systems. Bioconjugate Chem. 24 (7), 1210-1217 (2013).
- Rossin, R., Robillard, M. S. Pretargeted Imaging Using Bioorthogonal Chemistry in Mice. Curr. Opin. Chem. Biol. 21, 161-169 (2014).
- Denk, C., et al. Development of a 18F-Labeled Tetrazine with Favorable Pharmacokinetics for Bioorthogonal PET Imaging. Angew. Chem., Int. Ed. 53 (36), 9655-9659 (2014).
- Herth, M. M., Andersen, V. L., Lehel, S., Madsen, J., Knudsen, G. M., Kristensen, J. L. Development of a 11C-labeled Tetrazine for Rapid Tetrazine-Trans-Cyclooctene Ligation. Chem. Commun. 49 (36), 3805-3807 (2013).
- Li, Z., et al. Tetrazine-Trans-Cyclooctene Ligation for the Rapid Construction of 18F Labeled Probes. Chem. Commun. 46 (42), 8043 (2010).
- Nichols, B., Qin, Z., Yang, J., Vera, D. R., Devaraj, N. K. 68Ga Chelating Bioorthogonal Tetrazine Polymers for the Multistep Labeling of Cancer Biomarkers. Chem. Commun. 50 (40), 5215-5217 (2014).
- Zeglis, B. M., et al. A Pretargeted PET Imaging Strategy Based on Bioorthogonal Diels-Alder Click Chemistry. J. Nucl. Med. 54 (8), 1389-1396 (2013).
- García, M. F., et al. 99mTc-Bioorthogonal Click Chemistry Reagent for In Vivo Pretargeted Imaging. Bioorg. Med. Chem. 24 (6), 1209-1215 (2016).
- Russell, R. G. G. Bisphosphonates: The First 40 Years. Bone. 49 (1), 2-19 (2011).
- Yazdani, A., et al. A Bone-Seeking Trans-Cyclooctene for Pretargeting and Bioorthogonal Chemistry: A Proof of Concept Study Using 99mTc and 177Lu-Labeled Tetrazines. J. Med. Chem. , (2016).
- Alberto, R., et al. A Novel Organometallic Aqua Complex of Technetium for the Labeling of Biomolecules: Synthesis of [99mTc(OH2)3(CO)3]+ from [99mTcO4]- in Aqueous Solution and its Reaction with a Bifunctional Ligand. J. Am. Chem. Soc. 120 (31), 7987-7988 (1998).
- Alberto, R., Ortner, K., Wheatley, N., Schibli, R., Schubiger, A. P. Synthesis and properties of boranocarbonate: A convenient in situ CO source for the aqueous preparation of [99mTc(OH2)3(CO)3. J. Am. Chem. Soc. 123 (13), 3135-3136 (2001).
- Lu, G., et al. Synthesis and SAR of 99mTc/Re-labeled Small Molecule Prostate Specific Membrane Antigen Inhibitors with Novel Polar Chelates. Bioorg. Med. Chem. Lett. 23 (5), 1557-1563 (2013).
- Maresca, K. P., et al. Small Molecule Inhibitors of PSMA Incorporating Technetium-99m for Imaging Prostate Cancer: Effects of Chelate Design on Pharmacokinetics. Inorg. Chim. Acta. 389, 168-175 (2012).
- Bartholomä, M. D., et al. Insight into the Mode of Action of Re(CO)3 Thymidine Complexes. ChemMedChem. 5 (9), 1513-1529 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved