A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Glucose Uptake Measurement and Response to Insulin Stimulation in In Vitro Cultured Human Primary Myotubes
In This Article
Summary
In this method, human primary muscle cells are cultured in vitro to obtain differentiated myotubes and glucose uptake rates are measured. We provide a detailed protocol to quantify rates in basal and insulin-stimulated states using radiolabeled [3H] 2-deoxy-D-Glucose.
Abstract
Skeletal muscle is the largest glucose deposit in mammals and largely contributes to glucose homeostasis. Assessment of insulin sensitivity of muscle cells is of major relevance for all studies dedicated to exploring muscle glucose metabolism and characterizing metabolic alterations. In muscle cells, glucose transporter type 4 (GLUT4) proteins translocate to the plasma membrane in response to insulin, thus allowing massive entry of glucose into the cell. The ability of muscle cells to respond to insulin by increasing the rate of glucose uptake is one of the standard readouts to quantify muscle cell sensitivity to insulin. Human primary myotubes are a suitable in vitro model, as the cells maintain many features of the donor phenotype, including insulin sensitivity. This in vitro model is also suitable for the test of any compounds that could impact insulin responsiveness. Measurements of the glucose uptake rate in differentiated myotubes reflect insulin sensitivity.
In this method, human primary muscle cells are cultured in vitro to obtain differentiated myotubes, and glucose uptake rates with and without insulin stimulation are measured. We provide a detailed protocol to quantify passive and active glucose transport rates using radiolabeled [3H] 2-deoxy-D-Glucose ([3H]2dG). Calculation methods are provided to quantify active basal and insulin-stimulated rates, as well as stimulation fold.
Introduction
Skeletal muscle is the largest glucose deposit in mammals and largely contributes to glucose homeostasis. This insulin responsive tissue is the primary site of the glucose uptake that is triggered by insulin stimulation1.
In type 2 diabetes, insulin resistance is observed in several tissues, including skeletal muscle, and leads to above normal blood glucose concentration. Thus, it is of major relevance to determine the level of insulin sensitivity of this tissue and its cells, whether the aim is to characterize a defect in a subject, or to evaluate the efficiency of a treatment intending to improve it. In human or animal subjects, the gold standard technique to assess insulin sensitivity is the hyperinsulinemic-euglycemic clamp. Introduced by DeFronzo in 19792 and modified since3,4 then, the method allows to quantify whole body and tissues insulin responsiveness measured as the rate of glucose to be perfused under insulin stimulation to maintain normal blood glucose concentration.
Insulin sensitivity exploration can also be performed at the cell level using in vitro muscle models, and measurement of glucose uptake rates remains an efficient and reliable tool to quantify the biological response of the cell to insulin stimulation5,6,7. Indeed, glucose uptake measurement quantifies the cell biological response to insulin stimulation, from the binding of insulin to its receptor to translocation of GLUT4 enriched vesicles, and including intracellular signaling and phosphorylation cascades8.
This is of major interest with human samples, as differentiated myotubes maintain many features of the donor phenotype, including metabolic properties and disorders observed in the patient9,10,11,12. The myotubes displays structural, metabolic and phenotypic similarities to the skeletal muscle13,14, including the expression of glucose transporters15 and the cellular insulin signaling machinery16. Thus, measurement of the glucose uptake in primary myotubes is of relevance to characterizing the muscle phenotype of a donor, or investigating the effect of an intervention (drug, nutrition, or physical activity) on the insulin sensitivity in the muscle cell.
The measurement of glucose uptake on cultured myotubes also is a reliable tool when performing experiments that modify insulin sensitivity17,18. The in vitro model is suitable for the test of any compounds that could improve insulin responsiveness, or could prevent or reverse acquired or induced insulin resistance19,20,21,22,23.
Here we describe a detailed protocol to culture and differentiate human myotubes and to measure cell glucose uptake rates. The method is applicable to any source of human muscle precursor cells, whether they come from in-lab preparations, collaboration, or commercially available suppliers. Immortalized muscle cell lines, like C2C12 and L6, respectively from mouse and rat origin, can also be used for glucose uptake measurement with this protocol7.
We provide a detailed protocol to quantify rates in basal and insulin-stimulated states using radiolabeled [3H]2dG. The use of a labeled glucose analog allows accurate determination of glucose entry with reduced starting material, a common condition when working with primary cells. The modified glucose molecule is unable to enter metabolic pathways, and thus, accumulates within the cell, allowing reliable quantification via total cell radioactivity. Experimental conditions include the use of a glucose transport inhibitor (cytochalasin B), and measurements are performed with and without insulin. This combination allows the determination of glucose active entry rates, as well as the calculation of fold change for the insulin response index. The method is presented with one dose of insulin during a single incubation time, but the protocol can easily be modified for dose response or time course experiments12.
Protocol
1. Preparation of Cell Culture Media and Solutions
- Preparation of culture media
- Prepare proliferation medium (PM) by supplementing Ham's F-10 medium with glutamine (2 mM), penicillin/streptomycin (5 µg/mL final), 2% Fetal Calf Serum (FCS) and 2% serum substitute.
- Prepare differentiation medium (DM) by supplementing Dulbecco's Modified Eagle Medium (DMEM) with glutamine (2 mM), penicillin/streptomycin (5 µg/mL final), and 2% FCS.
- Preparation of glucose uptake solutions
Caution: Handling of radioactive material is only allowed in a restricted and controlled area by authorized personnel. Material and waste must be handled according to appropriate procedures, guidelines and local legislation.- To prepare X-Dulbecco's phosphate-buffered saline (X-DPBS), make a solution of DPBS containing 0.2% (w/v) (final concentration) bovine serum albumin (BSA). Filter the solution through a 0.2 µm filter. Store at 4 °C.
- To prepare cold 2-deoxy-D-glucose (2dG) solution, weigh 16.4 mg of 2dG and solubilize in 10 mL distilled water to obtain a 10 mM solution. Store at 4 °C.
- Add 600 µL of cold 2dG and 6 µL of radiolabeled [3H] 2dG to 5400 µL of X-DPBS to obtain the radiolabeled 2dG (2dG*) solution.
NOTE: The final concentration is 1 mM 2dG and labeling is 1 µCi/mL.- Set aside a 20 µL aliquot (TC20) of radiolabeled 2dG* solution.
- Preparation of incubation mixtures
- For the cytochalasin B mixture, add 2 µL of 20 mM cytochalasin B to 2 mL of radiolabeled 2dG* solution.
NOTE: Stock solution of cytochalasin B is at 10 mg/mL in dimethyl sulfoxide (DMSO). - For the DMSO mixture, add 4 µL of DMSO to the remaining 4 mL of radiolabeled 2dG* solution.
- For the cytochalasin B mixture, add 2 µL of 20 mM cytochalasin B to 2 mL of radiolabeled 2dG* solution.
2. Culture of Human Primary Muscle Cells
- Seeding of 6-well plates with human muscle satellite cells
NOTE: Use in-house (see reference 24 for details) or commercially available human muscle satellite cells from a frozen vial (containing 250,000 cells). The following procedure is given for 250,000 cells in order to obtain one 6-well plate necessary for the measurement of glucose uptake in a single condition.- Rapidly thaw frozen vials of in-house24 or commercial preparations of human muscle satellite cells in pre-warmed water (37 °C) until only a small ice block remains in the vial.
- Pour directly into a 50 mL plastic tube containing 10 mL of pre-warmed (37 °C) PM.
- Centrifuge for 5 min at 500 x g and discard the supernatant.
- Gently resuspend the cell pellet with 18 mL of pre-warmed PM (to obtain 42,000 cells per 3 mL of medium). Distribute 3 mL in each well of a 6-well plate (9.6 cm2).
NOTE: The six individual wells of a plate are required to perform a duplicate measurement of glucose uptake for the following conditions: passive transport inhibition (wells 1 and 2), basal rate (wells 3 and 4) and insulin stimulated rate (wells 5 and 6). Repeat as many six-well plates as distinct treatments are needed. - Incubate in standard culture conditions (37 °C, 5% CO2) until cells reach 90% confluence.
NOTE: This step takes between 48 - 72 h depending on the cell batch. Do not change medium during this step.
- Differentiation of muscle cells
- Remove PM (after 48-72 h) and replace with pre-warmed DM (3 mL per well). Incubate at 37 °C, 5% CO2.
NOTE: Differentiation takes five days to reach a stable state where cells are aligned and polynucleated. Typically, the primary myotubes are cultured in a 1 g/L glucose medium. Therefore, to avoid glucose depletion during culture, fill the plate with 3 mL medium to ensure that enough glucose substrate is available for the cells at all time. - Replace DM medium every two days.
NOTE: From this point, myotubes are stable for up to 7 days without any significant change and glucose uptake measurement can be performed at any time.
- Remove PM (after 48-72 h) and replace with pre-warmed DM (3 mL per well). Incubate at 37 °C, 5% CO2.
- Muscle cells treatment (optional)
NOTE: Primary myotubes can be treated for several days to induce modification (drug test, inhibitors/activators of signaling pathway, etc.) before insulin stimulation and glucose uptake measurements. Muscle cells can be submitted to any treatment that may have an impact on insulin sensitivity, and glucose uptake measurement will quantify this impact. For example, incubation of muscle cells with the saturated fatty acid palmitate promotes insulin resistance, and cells display reduced insulin stimulated glucose uptake.- Prepare 12 mL of DM supplemented with 10% BSA (fatty acid free) and 0.5 mL palmitate (PALM). Prepare 12 mL of DM supplemented with 10% BSA (fatty acid free) only.
- Prepare two 6-well plates with human primary myotubes, and culture them as described in sections 2.1 and 2.2 (with 5 days of differentiation).
- On day 5, wash each well with 2 mL of PBS. To one plate, add 2 mL of DM containing PALM. To the other plate add 2 mL of BSA only containing DM.
- Incubate for 48 h at 37 °C, 5% CO2.
3. Insulin Stimulation
- Wash differentiated muscle cells twice with 2 mL PBS.
- Remove PBS carefully and incubate with 3 mL of DM without FCS for 3 h (37 °C, 5% CO2) for serum depletion.
- Replace media in all wells with 3 mL of DM without FCS. Add 100 nM insulin to wells 5 and 6.
- Incubate human myotubes culture for 1 h (37 °C, 5% CO2).
4. Glucose Uptake
- After 1 h of insulin stimulation, wash wells twice with X-DPBS (1 mL per wash).
- Add 1 mL of cytochalasin B mixture to wells 1 and 2, and 1 mL of DMSO mixture to wells 3 - 6. Incubate for 15 min (37 °C, 5% CO2). At the end of the incubation, immediately place the plate on ice.
5. Cell Lysis
- Wash the cells twice with 1 mL of ice cold PBS.
- Lyse the cells in each well with 600 µL of 50 mM NaOH. Incubate on ice for 5 min and mix gently with slow orbital rotation.
NOTE: If the lysate is too viscous, dilute with up to 1.5 mL NaOH. - Using a pipette, resuspend and collect the cell lysate.
6. Determination of Radiolabeled Glucose
- Put 400 µL of each cell lysate in a liquid scintillation counting vial. Prepare a negative control vial with 400 µL of 50 mM NaOH, and a positive control vial with 20 µL of TC20 (from step 1.2.3.1).
- Add 4 mL of liquid scintillation solution to each vial. Close the cap and mix each vial thoroughly (1-2 s).
- Insert each vial in a liquid scintillation counter and measure the radioactivity according to the manufacturer's instruction. Record counts per min (CPM) for each scintillation vial for 10 min.
NOTE: CPM = "disintegrations per minute" x "counting efficiency".
7. Rate of Glucose Uptake
- Use the remaining lysate (200 µL; from step 5.2) to measure the protein concentration. Determine the protein concentration of each cell lysate using Bradford25 or an equivalent method. Calculate total protein quantity (Q) in mg for each well.
- To obtain TC1 (the value for 1 µL of radiolabeled 2dG*), divide the CPM value of TC20 by 20.
- For each vial, calculate the rate of glucose uptake as follows:
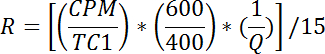
NOTE: R is measured in pmol/mg/min. Mean of R for wells 1 - 2 gives passive transport rate, Rp. Mean of R for wells 3-4 gives basal total transport rate, Rbt. Mean of R for wells 5-6 gives insulin stimulated total transport rate, Rit.- Calculate the basal active transport rate (Rba) as follows:
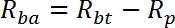
- Calculate the insulin stimulated active transport rate (Ria) as follows:
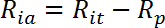
NOTE: In insulin responsive cells like myotubes, glucose uptake rates are usually represented by three values: Rba, Ria, and the fold insulin stimulation as Ria/Rba.
- Calculate the basal active transport rate (Rba) as follows:
Results
On day 3, myoblasts reach confluence (Figure 1A). The myoblasts at this stage are typically mononucleated. Medium was changed and on day 8, differentiation was completed (Figure 1B) (protocol section 2). After 5 days of differentiation, myotubes are aligned and typically polynucleated. Human primary myotubes were subjected to a palmitate or a BSA-only treatment before glucose uptake rate measurement. Cells were incubated for 48 h...
Discussion
Glucose uptake is a key biological measurement for testing activators or inhibitors on cell culture and how they impact glucose use, and the ability of the cell to respond to insulin. The method described here has been shown to be quick and reliable and has been widely used in many studies using primary myotubes from healthy subjects and/or metabolically affected patients6,7,10,12,
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge Anne Charrié at the Radiobiology service (Lyon-Sud hospital) and the Fond National Suisse (FNS) for their financial support.
Materials
| Name | Company | Catalog Number | Comments |
| Human primary muscle cell | In house preparation from human skeletal muscle biopsies | In house preparation from human skeletal muscle biopsies | If not available, use commercial source |
| Human primary muscle cell | Promocell | C-12530 | Should be cultured with associated media C23060 and C23061 |
| 6-well plate | Corning | 356400 | BioCoat Collagen I Multiwell Plates |
| Ham's F10 | Dutscher | L0145-500 | 1 g/L glucose |
| Glutamine | Dutscher | X0551-100 | |
| penicilin/streptomycin 100x | Thermo fisher scientific | 15140122 | |
| Serum substitute UltroserG | Pall France | 15950.017 | serum substitute in text |
| DMEM low glucose | Dutscher | L0064-500 | 1 g/L glucose |
| Fetal Calf Serum | Eurobio | CVFSVF00-01 | |
| Dulbecco's Phosphate-Buffered Saline | Dutscher | L0625-500 | Contains Mg2+ (0.5 mM) and Ca2+ (0.9 mM) |
| Insulin solution human | Sigma-Aldrich | I9278 | |
| 2-deoxy-D-glucose | Sigma-Aldrich | D6134 | |
| Albumin bovine | euromedex | 04-100-812-E | |
| fatty acid-free BSA | Roche | 10,775,835,001 | |
| palmitate | Sigma-Aldrich | P0500 | |
| Deoxy-D-glucose, 2-[1,2-3H (N)] | PerkinElmer | NET328A001MC | Specific Activity: 5 - 10 Ci (185-370GBq)/mmol, 1 mCi (37MBq |
| Cytochalasin B | Sigma-Aldrich | c2743 | |
| PICO PRIAS VIAL 6 mL | PerkinElmer | 6000192 | |
| ultima gold MW CA | PerkinElmer | 6013159 | scintillation liquid |
| bêta counter | PerkinElmer | 2900TR |
References
- Stump, C. S., Henriksen, E. J., Wei, Y., Sowers, J. R. The metabolic syndrome: role of skeletal muscle metabolism. Ann Med. 38 (6), 389-402 (2006).
- DeFronzo, R. A., Tobin, J. D., Andres, R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 237 (3), E214-E223 (1979).
- Fossum, E., Hoieggen, A., Moan, A., Nordby, G., Kjeldsen, S. E. Insulin sensitivity relates to other cardiovascular risk factors in young men: validation of some modifications of the hyperinsulinaemic, isoglycaemic glucose clamp technique. Blood Press Suppl. 2, 113-119 (1997).
- Heise, T., et al. Euglycaemic glucose clamp: what it can and cannot do, and how to do it. Diabetes Obes Metab. 18 (10), 962-972 (2016).
- Sell, H., Jensen, J., Eckel, J. Measurement of insulin sensitivity in skeletal muscle in vitro. Methods Mol Biol. 933, 255-263 (2012).
- Sarabia, V., Lam, L., Burdett, E., Leiter, L. A., Klip, A. Glucose transport in human skeletal muscle cells in culture. Stimulation by insulin and metformin. J Clin Invest. 90 (4), 1386-1395 (1992).
- Sarabia, V., Ramlal, T., Klip, A. Glucose uptake in human and animal muscle cells in culture. Biochem Cell Biol. 68 (2), 536-542 (1990).
- Richter, E. A., Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 93 (3), 993-1017 (2013).
- Gaster, M., Kristensen, S. R., Beck-Nielsen, H., Schroder, H. D. A cellular model system of differentiated human myotubes. Apmis. 109 (11), 735-744 (2001).
- Bouzakri, K., et al. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes. 52 (6), 1319-1325 (2003).
- Scheele, C., et al. Satellite cells derived from obese humans with type 2 diabetes and differentiated into myocytes in vitro exhibit abnormal response to IL-6. PLoS One. 7 (6), e39657 (2012).
- Jackson, S., et al. Decreased insulin responsiveness of glucose uptake in cultured human skeletal muscle cells from insulin-resistant nondiabetic relatives of type 2 diabetic families. Diabetes. 49 (7), 1169-1177 (2000).
- Aas, V., et al. Are cultured human myotubes far from home?. Cell Tissue Res. 354 (3), 671-682 (2013).
- Bakke, S. S., et al. Myotubes from severely obese type 2 diabetic subjects accumulate less lipids and show higher lipolytic rate than myotubes from severely obese non-diabetic subjects. PLoS One. 10 (3), e0119556 (2015).
- Stuart, C. A., et al. Hexose transporter mRNAs for GLUT4, GLUT5, and GLUT12 predominate in human muscle. Am J Physiol Endocrinol Metab. 291 (5), E1067-E1073 (2006).
- Al-Khalili, L., et al. Insulin action in cultured human skeletal muscle cells during differentiation: assessment of cell surface GLUT4 and GLUT1 content. Cell Mol Life Sci. 60 (5), 991-998 (2003).
- Tsuka, S., et al. Promotion of insulin-induced glucose uptake in C2C12 myotubes by osteocalcin. Biochem Biophys Res Commun. 459 (3), 437-442 (2015).
- Gorbunov, E. A., Nicoll, J., Myslivets, A. A., Kachaeva, E. V., Tarasov, S. A. Subetta Enhances Sensitivity of Human Muscle Cells to Insulin. Bull Exp Biol Med. 159 (4), 463-465 (2015).
- Breen, D. M., Sanli, T., Giacca, A., Tsiani, E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem Biophys Res Commun. 374 (1), 117-122 (2008).
- Pinnamaneni, S. K., Southgate, R. J., Febbraio, M. A., Watt, M. J. Stearoyl CoA desaturase 1 is elevated in obesity but protects against fatty acid-induced skeletal muscle insulin resistance in vitro. Diabetologia. 49 (12), 3027-3037 (2006).
- Gastebois, C., et al. Transition from physical activity to inactivity increases skeletal muscle miR-148b content and triggers insulin resistance. Physiol Rep. 4 (17), (2016).
- Naimi, M., Tsakiridis, T., Stamatatos, T. C., Alexandropoulos, D. I., Tsiani, E. Increased skeletal muscle glucose uptake by rosemary extract through AMPK activation. Appl Physiol Nutr Metab. 40 (4), 407-413 (2015).
- Feng, Y. Z., et al. PPARdelta activation in human myotubes increases mitochondrial fatty acid oxidative capacity and reduces glucose utilization by a switch in substrate preference. Arch Physiol Biochem. 120 (1), 12-21 (2014).
- Perrin, L., et al. Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion. Mol Metab. 4 (11), 834-845 (2015).
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72, 248-254 (1976).
- Bouzakri, K., et al. Malonyl CoenzymeA decarboxylase regulates lipid and glucose metabolism in human skeletal muscle. Diabetes. 57 (6), 1508-1516 (2008).
- Shemyakin, A., et al. Endothelin-1 reduces glucose uptake in human skeletal muscle in vivo and in vitro. Diabetes. 60 (8), 2061-2067 (2011).
- Alkhateeb, H., Chabowski, A., Glatz, J. F., Luiken, J. F., Bonen, A. Two phases of palmitate-induced insulin resistance in skeletal muscle: impaired GLUT4 translocation is followed by a reduced GLUT4 intrinsic activity. Am J Physiol Endocrinol Metab. 293 (3), E783-E793 (2007).
- Coll, T., et al. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem. 283 (17), 11107-11116 (2008).
- Gaster, M., Rustan, A. C., Beck-Nielsen, H. Differential utilization of saturated palmitate and unsaturated oleate: evidence from cultured myotubes. Diabetes. 54 (3), 648-656 (2005).
- Hage Hassan, R., et al. Endoplasmic reticulum stress does not mediate palmitate-induced insulin resistance in mouse and human muscle cells. Diabetologia. 55 (1), 204-214 (2012).
- Haghani, K., Pashaei, S., Vakili, S., Taheripak, G., Bakhtiyari, S. TNF-alpha knockdown alleviates palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Biochem Biophys Res Commun. 460 (4), 977-982 (2015).
- Hommelberg, P. P., et al. Palmitate-induced skeletal muscle insulin resistance does not require NF-kappaB activation. Cell Mol Life Sci. 68 (7), 1215-1225 (2011).
- Yang, M., et al. Saturated fatty acid palmitate-induced insulin resistance is accompanied with myotube loss and the impaired expression of health benefit myokine genes in C2C12 myotubes. Lipids Health Dis. 12, 104 (2013).
- Peng, G., et al. Oleate blocks palmitate-induced abnormal lipid distribution, endoplasmic reticulum expansion and stress, and insulin resistance in skeletal muscle. Endocrinology. 152 (6), 2206-2218 (2011).
- Lambernd, S., et al. Contractile activity of human skeletal muscle cells prevents insulin resistance by inhibiting pro-inflammatory signalling pathways. Diabetologia. 55 (4), 1128-1139 (2012).
- Nikolic, N., et al. Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLoS One. 7 (3), e33203 (2012).
- Hsu, F. L., et al. Antidiabetic effects of pterosin A, a small-molecular-weight natural product, on diabetic mouse models. Diabetes. 62 (2), 628-638 (2013).
- Zou, C., Wang, Y., Shen, Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J Biochem Biophys Methods. 64 (3), 207-215 (2005).
- Catalano, K. J., et al. Insulin resistance induced by hyperinsulinemia coincides with a persistent alteration at the insulin receptor tyrosine kinase domain. PLoS One. 9 (9), e108693 (2014).
- Liu, H. Y., et al. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM). J Biol Chem. 284 (40), 27090-27100 (2009).
- Renstrom, F., Buren, J., Svensson, M., Eriksson, J. W. Insulin resistance induced by high glucose and high insulin precedes insulin receptor substrate 1 protein depletion in human adipocytes. Metabolism. 56 (2), 190-198 (2007).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved