Summary
Abstract
Introduction
Protocol
Representative Results
Discussion
Acknowledgements
Materials
References
Neuroscience
Real-time Iontophoresis with Tetramethylammonium to Quantify Volume Fraction and Tortuosity of Brain Extracellular Space
This protocol describes real-time iontophoresis, a method that measures physical parameters of the extracellular space (ECS) of living brains. The diffusion of an inert molecule released into the ECS is used to calculate the ECS volume fraction and tortuosity. It is ideal for studying acute reversible changes to brain ECS.
This review describes the basic concepts and protocol to perform the real-time iontophoresis (RTI) method, the gold-standard to explore and quantify the extracellular space (ECS) of the living brain. The ECS surrounds all brain cells and contains both interstitial fluid and extracellular matrix. The transport of many substances required for brain activity, including neurotransmitters, hormones, and nutrients, occurs by diffusion through the ECS. Changes in the volume and geometry of this space occur during normal brain processes, like sleep, and pathological conditions, like ischemia. However, the structure and regulation of brain ECS, particularly in diseased states, remains largely unexplored. The RTI method measures two physical parameters of living brain: volume fraction and tortuosity. Volume fraction is the proportion of tissue volume occupied by ECS. Tortuosity is a measure of the relative hindrance a substance encounters when diffusing through a brain region as compared to a medium with no obstructions. In RTI, an inert molecule is pulsed from a source microelectrode into the brain ECS. As molecules diffuse away from this source, the changing concentration of the ion is measured over time using an ion-selective microelectrode positioned roughly 100 µm away. From the resulting diffusion curve, both volume fraction and tortuosity can be calculated. This technique has been used in brain slices from multiple species (including humans) and in vivo to study acute and chronic changes to ECS. Unlike other methods, RTI can be used to examine both reversible and irreversible changes to the brain ECS in real time.
The extracellular space (ECS) is the network of interconnected channels exterior to all brain cells and contains both interstitial fluid and extracellular matrix (Figure 1a and Figure 1b). The distribution of many substances required for brain cell function, including nutrients, hormones, and neurotransmitters, occurs by diffusion through the ECS. Changes in the physical parameters of this space, including volume, geometry, and extracellular matrix, can drastically affect diffusion through the ECS and the local ion concentrations bathing brain cells, which have a profound impact on brain cell function
All animal procedures, used to obtain tissue samples, were approval by the animal ethics committee at SUNY Downstate Medical Center.
1. Preparation of Solutions and Equipment
- Prepare a 150 mM NaCl backfill solution for the reference barrel of the ISM. Store it in a 10 mL syringe attached to a 0.22 µm filter (to remove bacteria or particles).
- Prepare a 150 mM TMA chloride (TMA-Cl) backfill solution for the microelectrodes. Store it in a 10 mL syringe attached to a 0.2.......
The utility of the RTI technique is demonstrated in an experiment designed to measure the changes in α and during a hypoosmolar challenge (Figure 8 and Figure 9). It has previously been shown that reducing the osmolarity of the ECS by washing on hypotonic ACSF will produce a decrease in α and an increase in λ13.
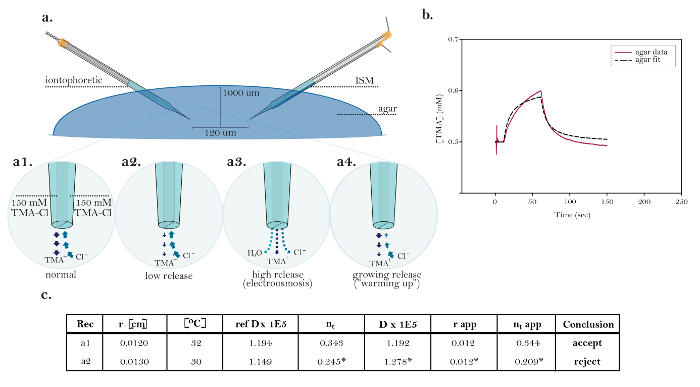
Figure 10: Non-ideal Data Demonstrating Common Technical Issues. (a) Diagrams of common technical issues with iontophoresis microelectrodes: Comparison of the normal release of TMA from a functioning iontophoresis microelectrode with three sources demonstrating technical issues. [High magnification, a1] The current in an ideal iontophoretic source is carried equally by TMA release and chloride upta.......
| Name | Company | Catalog Number | Comments |
| A/D and D/A converter | National Instruments Corporation | NI USB-6221 DAQ | The NI USB-6221 is still sold as a 'Legacy' device by NI. They recommend using NI USB-6341 X Series DAQs for new installations, however we have not tested the newer units. We describe the use of the NI USB-6221 with MATLAB and Windows 7 (32-bit). Alternatives: the much older PCI-MIO-16E-4 A/D converter (Used under Windows XP or older OS only) with BNC-2090 BNC connector panel and SH68-68-EP cable. As noted in the Wanda Manual, an experimental MATLAB program to use Axon Binary Files is available. |
| agarose | Lonza | NuSieve GTG Agarose #50081 | to prepare dilute agarose gel for RTI measurements |
| amplifier for ISM | Dagan | Model IX2-700 Dual Intracellular Preamplifier | ion and reference voltage amplifier with N=0.1 (for reference barrel) and N=0.001 (for ion barrel) headstages |
| biological compound miscroscope (with 4x and 10x objective) | for chipping the microelectrode tips and inspecting microelectrodes; various suppliers, e.g. AmScope | ||
| borosilicate theta capillary glass tubing | Harvard Apparatus | Warner Instruments model TG200-4; order #64-0811 | double-barreled glass tubing for ion-selective microelectrodes and iontophoretic microelectrodes; O.D. 2.0 mm, I.D. 1.4 mm, septum 0.2 mm, length 10 cm |
| brush | Winsor & Newton | University Series 233, size 0 | round shoft handle brush, available from Amazon |
| bunsen burner | Fisher | ||
| camera for visualizing micropipettes | Olympus | OLY-150 | requires monitor, IR filter on substage illuminator is optional |
| chart recorder | to record continuously voltages on ion-selective microelectrode during calibration in tetramethylammonium standards and during RTI experiment; e.g. Kipp & Zonen type BD112 dual-cannel chart recorded, available refurbished | ||
| chlorotrimethylsilane, puriss., > 99% | Sigma-Aldrich | catalog # 92360 | for silanization; CAUTION: flammable, acute toxicity (oral, dermal, inhalation), skin corrosion, eye damage, reacts violently with water, see Sigma-Aldrich Safety Information for full description |
| Commercial Software | The MathWorks | MATLAB, Data acquisition toolbox | for data acquisition and analysis using Wanda and Walter programs. Note that an academic license is available. |
| eye protective goggles | Fisher | ||
| fixed-stage compound microscope | Olympus | BX51WI | can use other compound microscopes with fixed stages |
| forceps | Fine Science Tools | #11251-10 | to chip glass capillary; Dumond #5, preferably used and no longer needed for fine work |
| fume hood | for silanization and filling the tip of ion-selective barrel with liquid ion exchanger; various supliers, e.g. Captair with approriate filter sold by Erlab | ||
| glass microscope slide | Fisher | #12-550A | to chip microelectrode tips |
| heater/stirrer | Fisher | Corning PC-420D | to prepare dilute agarose gel and stir solutions |
| iontophoretic unit | Dagan | ION-100 and PS-100 | ION-100 is a single channel iontophoresis unit +/- 130 V compliance; PS-100 is an external power supply; alternatives: e.g. Axoprobe-1A made by Axon Instruments (now Molecular Devices), out of production, check for availability of refurbished units (eBay and other sites) |
| liquid ion exchanger (LIX) for tetramethylammonium | World Precision Instruments | IE190 Potassium Ion Exchanger | Note: this is equivalent to the original Corning potassium exchanger 477317 based on tetraphenlyborate - do not confuse with neutral carrier potassium exchanger originating from the laboartory of Dr. Simon, ETH, Zurich, which does not sense tetramethylammonium, and is sold by Fluka. You can also make liquid ion exchanger for tetramethylammonium yourself: 3% by weight potassium tetrakis = (p-chlorophenyl) borate dissolved in 2,3-dimethylnitrobenzene. Buy chemicals from Fluka (now part of Sigma). See Oehme and Simon (1976) Anal. Chim. Acta 86: 21-25; CAUTION: The toxicological properties of this liquid ion exchanger have not been fully determined. Ingestion or contact with the human body may be harmful. Exercise due care! Liquid ion exchangers should be stored in a cool place out of direct sunlight. |
| microelectrode holder | WPI | M3301EH | to hold ion-selective microeletrode prefabricate for silanization and filling the tip of ion-selective barrel with liquid ion exchanger; WPI sells two versions of this holder, clear M3301EH and black M3301EH. In our experience, the clear M3301EH appears to be sturdier then the black M3301EH. |
| micromanipulator | Narishige | MM-3 | to position ion-selective microelectrode prefabricate during silanization and filling the tip of ion-selective barrel with liquid ion exchanger; can be substituted with any three-axis micromanipulator in good working condition |
| micropipette puller | Sutter Instruments | Model P-97 | to pull double-barreled glass tubing; other pullers can be used as long as they can accommodate large diameter double-barreled glass tubing |
| microprobe thermometer | Physiotemp | Model BAT-12R | fine probe of this thermometer is placed close to recording site |
| needle | BD | Syringes and Needles # 305122 (25 gauge) | for silanization; BD PrecisionGlide needles 25 G x 5/8 in (0.5mm x 16mm) |
| objective 5x dry | Olympus | MPlan N | |
| objective 10x water immersion | Olympus | UMPlan FL N | 10x objective is water immersion, numerical aperture is 0.3, working distance is 3.3 mm |
| plastic containers (with lids) | Fisher | #14-375-148 | to store tetramethylammonium standard solutions and microelectrodes |
| platform and x-y translation stage for fixed-stage microscope | EXFO | Gibraltar Burleigh | platform holds slice chamber, micromanipulators and accesorries, x-y translational stage moves microscope without compromising recording stability |
| porous minicup | for RTI measurements in a dilute agarose gel; homemade | ||
| reusable adhesive | Bostik | Blu-Tack | for securing microelectrodes to holding vessel and other uses; various suppliers, available from Amazon |
| robotic micromanipulator with precise x,y,z positioning | Sutter Instruments | MP-285 | two mircomanipulators are needed to hold separately ion-selective microelectrode and iontophoretic microelectrode. Also possible to glue micropipettes in a spaced array (see text). |
| signal conditioning unit with low-pass filter | Axon Instruments | CyberAmp 320 or 380 | no longer available from the manufacturer but may be available from E-Bay; alternatives: e.g. FLA-01 Filter/Amplifier from Cygnus Technology. This is a single channel instrument with a minimum cutoff at 10 Hz using a multipole Bessel filter but the company may be willing to modify it for a lower cutoff frequency (2 Hz) if needed. |
| silver wire | A-M Systems | #7830 | diameter 0.015", bare (no coating) |
| slice chamber | Harvard Apparatus | Warner Model RC-27L | this is submersion slice chamber; do not use interface slice chamber |
| stereomicroscope | for silanization and filling the tip of ion-selective barrel with liquid ion exchanger; horizontally mounted; various suppliers | ||
| syringe, 10 mL | BD | Syringes and Needles #309604 | to backfill microelectrodes and for silanization; BD Luer-Lok tip |
| syringe filter 0.22µm pore | Whatman | #6780-1302 | to filter backfill solutions; available from Fisher |
| syringe needle, 28 gauge, 97mm | World Precision Instruments | MicroFil MF28G-5 | to backfill microelectrodes |
| Teflon (=PTFE) tubing | Component Supply | STT-28 PTFE tube light wall (28 gauge) | for silanization of ion-selective barrel; fits on BD PrecisionGlide needles 25 G x 5/8 in. Note: Teflon is essential, PVC tubing would melt by hot wax. |
| temperature control system | Harvard Apparatus | Warner Models TC-344B and SH-27A | TC-344B is a dual automatic temperature controller, SH-27A is an in-line heater; controller and heater work with Warner slice chambers |
| tetramethyammonium (TMA) chloride | Sigma-Aldrich | T-3411 | 5 M solution; CAUTION: acute toxicity (oral, dermal, inhalation), carcinogenicity, hazardous to the aquatic environment, see Sigma-Aldrich Safety Information for full description |
| vibrating blade microtome | Leica | VT1000S | to cut brain slices |
| xylenes | Fisher | X5-1 | for silanization; CAUTION: flammable, acute toxicity (oral, dermal, inhalation), skin corrosion, eye damage, carcinogenicity, see Fisher Safety Information for full description |
- Sykova, E., Nicholson, C. Diffusion in brain extracellular space. Physiol Rev. 88 (4), 1277-1340 (2008).
- Nicholson, C. Diffusion and related transport mechanisms in brain tissue. Rep Prog Phys. 64 (7), 815-884 (2001).
- Nicholson, C. Ion-selective microelectrodes and diffusion measurements as tools to explore the brain cell microenvironment. J Neurosci Methods. 48 (3), 199-213 (1993).
- Nicholson, C., Phillips, J. M. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol. 321, 225-257 (1981).
- Nicholson, C., Sykova, E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 21 (5), 207-215 (1998).
- Xie, L. L., et al. Sleep drives metabolite clearance from the adult brain. Science. 342 (6156), 373-377 (2013).
- Hrabetova, S., Nicholson, C., Michael, A. C., Borland, L. M. Biophysical properties of brain extracellular space explored with ion-selective microelectrodes, integrative optical imaging and related techniques. Electrochemical Methods for Neuroscience Neuroscience. , 167-204 (2007).
- Rice, M. E., Okada, Y. C., Nicholson, C. Anisotropic and heterogeneous diffusion in the turtle cerebellum: implications for volume transmission. J Neurophysiol. 70 (5), 2035-2044 (1993).
- Vargova, L., et al. Diffusion parameters of the extracellular space in human gliomas. Glia. 42 (1), 77-88 (2003).
- Haack, N., Durry, S., Kafitz, K. W., Chesler, M., Rose, C. Double-barreled and concentric microelectrodes for measurement of extracellular ion signals in brain tissue. J Vis Exp. (103), (2015).
- Xiao, F., Hrabetova, S. Enlarged extracellular space of aquaporin-4-deficient mice does not enhance diffusion of Alexa Fluor 488 or dextran polymers. Neuroscience. 161 (1), 39-45 (2009).
- Sherpa, A. D., Pvan de Nes, ., Xiao, F., Weedon, J., Hrabetova, S. Gliotoxin-induced swelling of astrocytes hinders diffusion in brain extracellular space via formation of dead-space microdomains. Glia. 62 (7), 1053-1065 (2014).
- Kume-Kick, J., et al. Independence of extracellular tortuosity and volume fraction during osmotic challenge in rat neocortex. J Physiol. 542 (Pt 2), 515-527 (2002).
- Saghyan, A., Lewis, D. P., Hrabe, J., Hrabetova, S. Extracellular diffusion in laminar brain structures exemplified by hippocampus. J Neurosci Methods. 205 (1), 110-118 (2012).
- Fedirko, N., Svichar, N., Chesler, M. Fabrication and use of high-speed, concentric H+- and Ca2+-selective microelectrodes suitable for in vitro extracellular recording. J Neurophys. 96 (2), 919-924 (2006).
- Nicholson, C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 333 (2), 325-329 (1985).
- Nicholson, C., Tao, L. Hindered diffusion of high molecular weight compounds in brain extracellular microenvironment measured with integrative optical imaging. Biophys J. 65 (6), 2277-2290 (1993).
- Thorne, R. G., Nicholson, C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci U S A. 103 (14), 5567-5572 (2006).
- Wolak, D. J., Thorne, R. G. Diffusion of macromolecules in the brain: implications for drug delivery. Mol Pharm. 10 (5), 1492-1504 (2013).
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved