A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Microfluidic Devices for Characterizing Pore-scale Event Processes in Porous Media for Oil Recovery Applications
* These authors contributed equally
In This Article
Summary
The goal of this procedure is to easily and rapidly produce a microfluidic device with customizable geometry and resistance to swelling by organic fluids for oil recovery studies. A polydimethylsiloxane mold is first generated, and then used to cast the epoxy-based device. A representative displacement study is reported.
Abstract
Microfluidic devices are versatile tools for studying transport processes at a microscopic scale. A demand exists for microfluidic devices that are resistant to low molecular-weight oil components, unlike traditional polydimethylsiloxane (PDMS) devices. Here, we demonstrate a facile method for making a device with this property, and we use the product of this protocol for examining the pore-scale mechanisms by which foam recovers crude oil. A pattern is first designed using computer-aided design (CAD) software and printed on a transparency with a high-resolution printer. This pattern is then transferred to a photoresist via a lithography procedure. PDMS is cast on the pattern, cured in an oven, and removed to obtain a mold. A thiol-ene crosslinking polymer, commonly used as an optical adhesive (OA), is then poured onto the mold and cured under UV light. The PDMS mold is peeled away from the optical adhesive cast. A glass substrate is then prepared, and the two halves of the device are bonded together. Optical adhesive-based devices are more robust than traditional PDMS microfluidic devices. The epoxy structure is resistant to swelling by many organic solvents, which opens new possibilities for experiments involving light organic liquids. Additionally, the surface wettability behavior of these devices is more stable than that of PDMS. The construction of optical adhesive microfluidic devices is simple, yet requires incrementally more effort than the making of PDMS-based devices. Also, though optical adhesive devices are stable in organic liquids, they may exhibit reduced bond-strength after a long time. Optical adhesive microfluidic devices can be made in geometries that act as 2-D micromodels for porous media. These devices are applied in the study of oil displacement to improve our understanding of the pore-scale mechanisms involved in enhanced oil recovery and aquifer remediation.
Introduction
The purpose of this method is to visualize and analyze multi-phase, multi-component fluid interactions and complex pore-scale dynamics in porous media. Fluid flow and transport in porous media have been of interest for many years because these systems are applicable to several subsurface processes such as oil recovery, aquifer remediation, and hydraulic fracturing1,2,3,4,5. Using micromodels to mimic these complex pore-structures, unique insights are gained by visualizing pore-level dynamic events between the different fluid phases and the media6,7,8,9,10,11.
The fabrication of traditional silica-based micromodels is expensive, time consuming, and challenging, yet constructing micromodels from optical adhesive offers a relatively inexpensive, fast, and easy alternative12,13,14,15. Compared with other polymer-based micromodels, optical adhesive exhibits more stable surface wetting properties. For example, polydimethylsiloxane (PDMS) micromodel surfaces will quickly become hydrophobic during the course of a typical displacement experiment16. Furthermore, the Young's modulus of PDMS is 2.5 MPa whereas that of optical adhesive is 325 MPa13,17,18. Thus, optical adhesive is less prone to pressure induced deformation and channel failure. Importantly, cured optical adhesive is much more resistant to swelling by low molecular weight organic components, which allows experiments involving crude oil and light solvents to be conducted18. Overall, optical adhesive is a superior alternative to PDMS for displacement studies involving crude oil when silica-based micromodels are prohibitively complex or expensive and high temperature and pressure studies are not required.
The protocol described in this publication provides the step-by-step fabrication instructions for optical adhesive micromodels and reports the subtle tricks that ensure success in the manipulation of small quantities of fluids. The design and fabrication of optical adhesive based micromodels with soft lithography is first described. Then, the fluid displacement strategy is given for ultra-low flow rates that are commonly unattainable with mass flow controllers. Next, a representative experimental result is given as an example. This experiment reveals foam destabilization and propagation behavior in the presence of crude oil and heterogeneous porous media. Lastly, typical image processing and data analysis is reported.
The method provided here is appropriate for visualization applications involving multi-phase flow and interactions in confined microchannel spaces. Specifically, this method is optimized for characteristic micro-feature resolutions greater than 5 and less than 700 µm. Typical flow rates are on the order of 0.1 to 1 mL/h. In studies of crude oil or light solvent displacement by aqueous or gaseous fluids on the order of these optimized parameters at ambient conditions, this protocol should be appropriate.
Access restricted. Please log in or start a trial to view this content.
Protocol
Caution: This protocol involves handling a high temperature oven, toxic chemicals, and UV light. Please read all the material safety data sheets carefully and follow your institution's chemical safety guidelines.
1. Device Design
- Design a photomask in a CAD software application.
- Draw a rectangular channel that is 3 cm long and 0.5 cm wide (Figure 1b-top right).
- Create an array of enclosed shapes representing the grains of the porous media.
NOTE: These shapes are referred to as posts because they will become three-dimensional structures during the soft lithography process. The shape and size of the posts should be on the order of tens of microns, and have a spacing of ten to one hundred microns. Multiple post sizes may be employed to create heterogeneity, and a section can be left bare of posts to simulate a fracture in the media. - Draw inlet and outlet channels that are approximately one-third as wide as the porous media section. Draw a channel stemming from the inlet port to act as a drain.
- Draw a bounding box around the entire design with a minimum of 1.0 cm of clearance from the design.
NOTE: The area between the bounding box and the borders of the design, as well as the posts, are to be made transparent on the photomask.
- Submit the CAD file to a company for high-resolution CAD printing
NOTE: Optional: For a foam displacement experiment, design a microfluidic foam generator (Figure 1a). Repeat step 1, omitting the design heterogeneity and bounding box. A flow-focusing geometry is recommended at the inlet before the porous media design. The flow spaces should be transparent on the photomask.
2. PDMS Mold Fabrication
- Create a photoresist-patterned silicon wafer master mold in a clean room
- Spin-coat a 20 µm layer of photoresist onto a new silicon wafer at 2,000 rpm for 30 s.
- Soft bake the wafer on a hot plate in two increments: 65 °C for 1 min followed by 95 °C for 3 min.
- Use a mask aligner to pattern the photoresist layer with the CAD design using a constant dosage of 150 mJ/cm2.
- Perform a post-exposure bake on a hot plate in two increments: 65 °C for 1 min followed by 95 °C for 3 min. Allow the wafer to cool for 5 min.
- Immerse the wafer in 100 mL of propylene-glycol-methyl-ether-acetate in a glass crystallization dish. Gently agitate by hand for 10 min to develop the photoresist pattern. Rinse it with isopropanol and dry the wafer under a stream of dry air.
- Hard bake the wafer on a hot plate in two increments: 120 °C for 5 min followed by 150 °C for 10 min. Allow the wafer to cool for 15 min.
- Cast PDMS onto the silicon wafer master mold
- Mix a total of 30 g of the PDMS elastomer and curing agent in a 5:1 ratio inside a dust-free disposable container.
- Degas the PDMS in a vacuum desiccator for 30 min.
- Pour the PDMS onto the photoresist-patterned silicon wafer master mold in a 150 mm glass Petri dish.
- Place the Petri dish containing the wafer and PDMS in an 80 °C oven for 1 h.
- Remove the Petri dish from the oven and allow the contents to reach room temperature.
NOTE: The procedure may be paused at this point.
- Prepare the PDMS mold for pattern transfer to optical adhesive
- Carefully cut the PDMS mold out using a scalpel, and peel the mold away from the wafer.
- Clean and protect the PDMS mold using clear adhesive tape.
NOTE: The procedure may be paused at this point. - Place the PDMS mold, pattern-side up, in the bottom of a dust-free 60 mm plastic Petri dish. Allow 10 s for the PDMS to stick to the plastic.
- Protect the surface of the PDMS with clear plastic tape until step 3.1.1.
NOTE: Optional: To make the foam generator, repeat steps 2.1. through 2.3.2. for the foam-generator design.
3. Optical Adhesive Device Fabrication
- Cast optical adhesive onto the PDMS mold
- Remove the tape from the patterned surface of the PDMS, and pour optical adhesive into the 150 mm Petri dish to a depth of approximately 0.9 cm above the top surface of the PDMS mold. Gently remove any bubbles with any type of cotton swab.
- Cure the optical adhesive under UV light for a total of 40 min as outlined in steps 3.2.1 - 3.2.5 in a PSD-UV system.
Caution: Wear appropriate protection when working with UV light.- Expose the Petri dish to UV light (254 nm) for 5 min.
- Invert the Petri dish such that the bottom is now facing the UV source, and expose the under-side to UV light for 5 min.
- Invert the Petri dish, return it to the upright position, and re-expose the top side to UV light for 5 min.
- Invert the Petri dish upside down again, and re-expose the bottom side to UV light for 10 min.
- Invert the Petri dish back to the upright position, and re-expose the top side to UV light for 15 min.
NOTE: The curing procedure in steps 3.2.1 through 3.2.5 is only applicable when the specified PSD-UV apparatus is used (Table of Materials). Cure times will vary depending on the specific lamp that is used and on the exact thickness of the optical adhesive layer.
- Remove the cured optical adhesive from the PDMS mold
- Use a box cutter to carefully break the optical adhesive out of the Petri dish mold.
Caution: Box cutter blades are very sharp and can easily cut flesh. Be careful when working around the sharp edges of broken Petri dishes. - Use a sturdy pair of scissors to remove excess optical adhesive from the edge of the design.
- Slowly peel the PDMS mold away from the optical adhesive puck. Protect the patterned portions of the optical adhesive surface and the PDMS surface with clear tape.
- Use a 1.0 mm biopsy punch to create inlet, outlet, and drain holes. Protect the patterned optical adhesive with clear tape.
- Use a box cutter to carefully break the optical adhesive out of the Petri dish mold.
- Prepare the substrate
- Dispense 1 mL of optical adhesive onto a new glass slide, and spin-coat the slide in two steps: 500 rpm for 5 s then 4,000 rpm for 20 s.
- Quickly transfer the substrate to the UV light treatment, and partially cure the thin optical adhesive layer under UV light for 30 s.
- Bond the optical adhesive cast to the substrate
- Place the optical adhesive cast, patterned side up, and the substrate, coated-side up, in an O2 plasma cleaner. Plasma clean the surface for 20 s at 540 mTorr.
- Firmly press the two treated surfaces together until all undesired air pockets have been minimized or removed.
- Fully cure the device under UV light for 20 min.
Caution: For UV light, wear appropriate protection such as protective glasses, lab coat, gloves, etc. - Place the device on a hot plate at 50 °C for 18 h.
- Insert a 6-inch long segment of 0.58 mm ID low density polyethylene tubing (PE/3) into each of the ports on the device.
- Use a 5 min quick-set epoxy to secure the tubing in place.
NOTE: Optional: To complete the foam generator, repeat steps 3.5.1, 3.5.2, 3.6, and 3.7. using the foam-generator PDMS cast and a new glass slide, instead of the optical adhesive cast and prepared substrates, respectively.
4. Oil Displacement Experiment
- Prepare the microfluidic device to be imaged on an inverted microscope equipped with a high-speed camera. Fix the device to the microscope stage using tape. Using a 4X objective, focus on the area of interest (AOI).
- Prepare the injection fluids
NOTE: For three-phase systems, a dye should be added to clear displacing fluids to provide color contrast for image analysis.- Load 3 mL of crude oil or model oil sample into a 10 mL glass syringe equipped with a 23 gauge industrial dispensing tip. Secure the syringe in the syringe pump holder and set the appropriate diameter value on the syringe pump settings.
- Load 1 mL of the displacing fluid into a 3 mL plastic syringe equipped with a 23 gauge industrial dispensing tip. Secure the syringe in the syringe pump holder and set the appropriate diameter value on the syringe pump settings.
NOTE: Optional: For foam generation experiments, connect a 10 m long 25 µm diameter glass capillary tube to an N2 gas tank and set the gas pressure to the desired value for the required gas flow rate as obtained from a calibration curve. Allow 10 min for the gas flow to equilibrate.
- Saturate the OPTICAL ADHESIVE model porous media device with oil
- Connect the displacing fluid to the inlet of the device by inserting the needle tip into the PE/3 tubing.
NOTE: Optional: When foam is used as the displacing phase, connect the displacing fluid syringe to the inlet of the foam generator. Connect the gas capillary to the second inlet port on the foam generator by inserting the capillary tube into a 23-gauge industrial dispensing tip and sealing the annulus with quick-set epoxy. The outlet of the foam generator is then connected to the inlet of the optical adhesive device using a 23-gauge connector. - Connect the oil-filled syringe to the inlet of the device by inserting the needle tip into the PE/3 tubing.
- Begin flowing the oil into the outlet port of the OPTICAL ADHESIVE device at 2 mL/h while simultaneously flowing the displacing fluid into the inlet port at 0.8 mL/h such that the two fluids both flow out the drain port. The displacing fluid should not enter the porous media. Collect the effluent in a 20 mL glass vial.
- Connect the displacing fluid to the inlet of the device by inserting the needle tip into the PE/3 tubing.
- Begin filming the AOI on the porous media device at a frame rate fast enough to capture the desired phenomena. A typical frame rate is 50 fps. Capture a still image of the 100% oil-saturated area.
- Swiftly and simultaneously cut the PE/3 tubing that is flowing in the oil using scissors while clamping the drain tube with a 5 cm binder clamp.
- Allow the displacing fluid to invade the device until either the oil displacement reaches steady-state or the camera runs out of memory.
5. Image and Data Analysis
- Use a free image analysis software such as Image J or use the image analysis toolbox in MATLAB to analyze the footage from the experiment.
- Using the still image of the 100% oil-saturated channel, calculate the porosity in units of percent for the porous media AOI.
- Calculate the pore volume using the following equation:
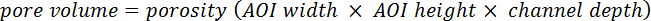
- Use image analysis software to determine the oil saturation, as a fraction of the total flow space, in each frame of the video footage from the experiment. For two phase displacement experiments, the displacing phase saturation in each frame may be calculated as:
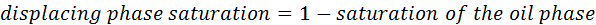
- Prepare a plot of oil saturation in percent vs. pore volumes of injected fluid
NOTE: Optional: For three phase systems such as those of foam displacement experiments, use the MATLAB image analysis toolbox to categorize each displacing phase by color using the characteristic RGB range for each phase. Prepare a plot showing the saturations of all three phases with injected pore volumes.
Access restricted. Please log in or start a trial to view this content.
Results
In this example experiment, aqueous foam is used to displace Middle East crude oil (with a viscosity of 5.4 cP and API gravity of 40°) in a heterogeneous porous media with layered permeability contrast. A PDMS foam generator is connected to an optical adhesive micromodel which was previously completely saturated with crude oil. Figure 1a shows the CAD design of the photomask for the PDMS foam generator, the photoresist-patterned silicon wafer, and the co...
Access restricted. Please log in or start a trial to view this content.
Discussion
This protocol for studying oil recovery processes in optical adhesive micromodels strikes a balance between the robustness of non-polymeric micromodels – such as glass or silicon – and the facile fabrication of PDMS microfluidic devices. Unlike micromodels made of glass or optical adhesive, PDMS devices lack resistance to light organic species. PDMS micromodels are also not ideal for many experiments because the surfaces of these devices have unstable wetting properties, and the polymer matrix is permeable to...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We acknowledge the financial support from the Rice University Consortium for Processes in Porous Media (Houston, TX, USA).
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 3 mL Leur-Lok Syringe | Fischer Scientific | 14-823-435 | |
| 10 mL Glass Syringe | Fischer Scientific | 1482698G | |
| Photomask | CAD/Art Services | ||
| Silicon Wafer | University Wafer | 452 | |
| Propylene-Glycol-Methyl-Ether-Acetate | Sigma Aldrich | 484431-4L | |
| 150 mm Glass Petri Dish | Carolina Biological Supply | #721134 | |
| 60 mm Plastic Petri Dish | Carolina Biological Supply | #741246 | |
| Mask Aligner | EV Group | EVG 620 | |
| 1 mm Biopsy Punch | Miltex, Plainsboro, NJ | 69031-01 | |
| Industrial Dispensing Tip | CML Supply | Gauge 23 | |
| Inverted Microscope | Olympus | IX-71 | |
| Plasma System | Harrick Plasma | PDC-32G | Plasma cleaner |
| Polydimehtylsiloxane (PDMS) | Dow Corning, Midland, MI | SYLGARD 184 | |
| Norland Optical Adhesive 81 (NOA81) or (OA) | Norland Products Inc. | 8116 | Optical adhesive |
| Quick-Set Epoxy | Fisher Scientific | 4001 | |
| Glass Slides | Globe Scientic Inc. | 1321 | |
| SU-8 2015 Photoresist | MicroChem | SU-8 2015 | Photo resist |
| Syringe Pump | Harvard Apparatus | Fusion 400 | |
| Glass Capillary Tubing | SGE Analytical Science | 1154710C | |
| High-Speed Camera | Vision Research | V 4.3 | |
| Polyethylene Tubing | Scientific Commodities Inc. | #BB31695-PE/3 |
References
- Blaker, T., et al. Foam for Gas Mobility Control in the Snorre Field: The FAWAG Project. SPE Reserv Eval Eng. 5 (04), 317-323 (2002).
- Mannhardt, K., Svorstøl, I. Effect of oil saturation on foam propagation in Snorre reservoir core. J Petrol Sci Eng. 23 (3-4), 189-200 (1999).
- Falls, A. H., Lawson, J. B., Hirasaki, G. J. The Role of Noncondensable Gas in Steam Foams. J Petrol Technol. 40 (01), 95-104 (1988).
- Hirasaki, G. J., Miller, C. A., Szafranski, R., Lawson, J. B., Akiya, N. Surfactant/Foam Process for Aquifer Remediation. , International Symposium on Oilfield Chemistry. Houston, Texas. (1997).
- Lv, Q., Li, Z., Li, B., Li, S., Sun, Q. Study of Nanoparticle-Surfactant-Stabilized Foam as a Fracturing Fluid. Ind Eng Chem Res. 54 (38), 9468-9477 (2015).
- Conn, C. A., Ma, K., Hirasaki, G. J., Biswal, S. L. Visualizing oil displacement with foam in a microfluidic device with permeability contrast. Lab Chip. 14 (20), 3968-3977 (2014).
- Ma, K., Liontas, R., Conn, C. A., Hirasaki, G. J., Biswal, S. L. Visualization of improved sweep with foam in heterogeneous porous media using microfluidics. Soft Matter. 8 (41), 10669(2012).
- Anna, S. L., Bontoux, N., Stone, H. A. Formation of dispersions using "flow focusing" in microchannels. Appl Phys Lett. 82 (3), 364(2003).
- Gauteplass, J., Chaudhary, K., Kovscek, A. R., Fernø, M. A. Pore-level foam generation and flow for mobility control in fractured systems. Colloid Surface A. 468, 184-192 (2015).
- Kovscek, A. R., Radke, C. J. Gas bubble snap-off under pressure-driven flow in constricted noncircular capillaries. Colloid Surface A. 117 (1-2), 55-76 (1996).
- Géraud, B., Jones, S. A., Cantat, I., Dollet, B., Méheust, Y. The flow of a foam in a two-dimensional porous medium: FOAM FLOW IN A 2-D POROUS MEDIUM. Water Resour Res. 52 (2), 773-790 (2016).
- Lin, Y. -J., et al. Examining Asphaltene Solubility on Deposition in Model Porous Media. Langmuir. 32 (34), 8729-8734 (2016).
- Bartolo, D., Degré, G., Nghe, P., Studer, V. Microfluidic stickers. Lab Chip. 8 (2), 274-279 (2008).
- Kenzhekhanov, S. Chemical EOR process visualization using NOA81 micromodels. , Master's degree Thesis (2017).
- Zhuang, Y. G., et al. Experimental Investigation of Asphaltene Deposition in a Transparent Microchannel. Proceedings of the 1st Thermal and Fluid Engineering Summer Conference. , New York, NY, USA. (2016).
- Ma, K., Rivera, J., Hirasaki, G. J., Biswal, S. L. Wettability control and patterning of PDMS using UV-ozone and water immersion. J Colloid Interf Sci. 363 (1), 371-378 (2011).
- Duffy, D. C., McDonald, J. C., Schueller, O. J. A., Whitesides, G. M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal Chem. 70 (23), 4974-4984 (1998).
- Sollier, E., Murray, C., Maoddi, P., Di Carlo, D. Rapid prototyping polymers for microfluidic devices and high pressure injections. Lab Chip. 11 (22), 3752(2011).
- Lee, J. N., Park, C., Whitesides, G. M. Solvent Compatibility of Poly(dimethylsiloxane)-Based Microfluidic Devices. Anal Chem. 75 (23), 6544-6554 (2003).
- Silvestrini, S., et al. Tailoring the wetting properties of thiolene microfluidic materials. Lab Chip. 12 (20), 4041(2012).
- Wägli, P., Homsy, A., de Rooij, N. F. Norland optical adhesive (NOA81) microchannels with adjustable wetting behavior and high chemical resistance against a range of mid-infrared-transparent organic solvents. Sensor Actuat B-Chem. 156 (2), 994-1001 (2011).
- Hung, L. -H., Lin, R., Lee, A. P. Rapid microfabrication of solvent-resistant biocompatible microfluidic devices. Lab Chip. 8 (6), 983(2008).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved