A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Investigating the Detrimental Effects of Low Pressure Plasma Sterilization on the Survival of Bacillus subtilis Spores Using Live Cell Microscopy
In This Article
Summary
This protocol illustrates the important consecutive steps required to assess the relevance of monitoring vitality parameter and DNA repair processes in reviving Bacillus subtilis spores after treatment with low pressure plasma by tracking fluorescence-labelled DNA repair proteins via time-resolved confocal microscopy and scanning electron microscopy.
Abstract
Plasma sterilization is a promising alternative to conventional sterilization methods for industrial, clinical, and spaceflight purposes. Low pressure plasma (LPP) discharges contain a broad spectrum of active species, which lead to rapid microbial inactivation. To study the efficiency and mechanisms of sterilization by LPP, we use spores of the test organism Bacillus subtilis because of their extraordinary resistance against conventional sterilization procedures. We describe the production of B. subtilis spore monolayers, the sterilization process by low pressure plasma in a double inductively coupled plasma reactor, the characterization of spore morphology using scanning electron microscopy (SEM), and the analysis of germination and outgrowth of spores by live cell microscopy. A major target of plasma species is genomic material (DNA) and repair of plasma-induced DNA lesions upon spore revival is crucial for survival of the organism. Here, we study the germination capacity of spores and the role of DNA repair during spore germination and outgrowth after treatment with LPP by tracking fluorescently-labelled DNA repair proteins (RecA) with time-resolved confocal fluorescence microscopy. Treated and untreated spore monolayers are activated for germination and visualized with an inverted confocal live cell microscope over time to follow the reaction of individual spores. Our observations reveal that the fraction of germinating and outgrowing spores is dependent on the duration of LPP-treatment reaching a minimum after 120 s. RecA-YFP (yellow fluorescence protein) fluorescence was detected only in few spores and developed in all outgrowing cells with a slight elevation in LPP-treated spores. Moreover, some of the vegetative bacteria derived from LPP-treated spores showed an increase in cytoplasm and tended to lyse. The described methods for analysis of individual spores could be exemplary for the study of other aspects of spore germination and outgrowth.
Introduction
A major goal of space exploration is the search for signatures of life forms and biomolecules on other planetary bodies and moons in our solar system. The transfer of microorganisms or biomolecules of terrestrial origin to critical areas of exploration is of particular risk to impact the development and integrity of life-detection missions on planetary bodies such as Mars and Europa1. The international guidelines of planetary protection, established by the Committee of Space Research (COSPAR) in 1967, impose strict regulations on manned and robotic missions to other planets, their moons, asteroids, and other celestial bodies and regulate the cleaning and sterilization of a spacecraft and critical hardware components prior to launch in order to eliminate contaminating terrestrial microorganisms and prevent cross contamination of celestial bodies2. Over the last decade, the application of non-thermal plasmas has gained wide attention in biomedical and nutritional research, as well as in spaceflight applications3,4,5. Plasma sterilization is a promising alternative to conventional sterilization methods as it offers rapid and efficient microbial inactivation6, while being gentle to sensitive and heat labile materials. Plasma discharges contain a mixture of reactive agents such as free radicals, charged particles, neutral/excited atoms, photons in the ultraviolet (UV), and vacuum ultraviolet (VUV) spectrum which lead to rapid microbial inactivation3. In this study, we use low-pressure plasma generated by double inductively coupled low-pressure plasma (DICP) source7,8 to inactivate Bacillus subtilis endospores distributed on glass test surface.
Gram-positive bacteria of the family Bacillaceae are widely distributed in natural habitats of soil, sediments, and air as well as in unusual environments such as clean room facilities and the International Space Station9,10,11. The most distinct feature of the genus Bacillus is the ability to form highly resistant dormant endospores (hereafter referred to as spores) to survive unfavorable conditions, such as nutrient depletion12. Spores are generally much more resistant than their vegetative cell counterparts to a variety of treatments and environmental stresses, including heat, UV, gamma irradiation, desiccation, mechanical disruption, and toxic chemicals, such as strong oxidizers or pH-changing agents (reviewed in references13,14) and are therefore ideal objects for testing the efficiency of microbial inactivation methods. Since genomic DNA is a major target of the plasma treatment of bacteria15,16, the repair of plasma-induced DNA lesions (e.g. DNA double strand breaks) upon spore revival is crucial for survival of bacteria13,17.
Thus, we study the germination capacity of spores and the role of DNA repair during spore germination and outgrowth after treating the spores with low pressure argon plasma by following individual spores and their expression of fluorescence-labelled DNA repair protein RecA with time-resolved confocal fluorescence microscopy. We give a step by step instruction of the preparation of B. subtilis spores in monolayers for achieving reproducible test results, the treatment of spore monolayers with low pressure plasma for sterilization, the preparation of plasma treated spores for ultrastructural evaluation using scanning electron microscopy (SEM), and live cell microscopy analysis at the level of individual spores in concert with monitoring the active DNA repair processes occurring within the cell in response to plasma treatment.
Protocol
1. Bacillus subtilis Spore Production and Purification
- For spore production, transfer a 5 mL overnight culture of the respective B. subtilis strain, supplemented with appropriate antibiotics, to 200 mL double-strength liquid Schaeffer sporulation medium (per liter 16 g nutrient broth, KCl 2 g, 0.5 g MgSO4*7 H2O, 2 mL 1 M Ca(NO3)2, 2 mL 0.1 M MnCl2 *• 4 H2O, 2 mL 1 mM FeSO4, 2 mL 50% (w/v) glucose18) and cultivate it with vigorous aeration at 37 °C for 72 h or until > 95% of the culture has sporulated. The spores of following strains are used: B. subtilis PY79 (wild type) B. subtilis PY79ΔrecA::neo (deficient of DNA repair protein RecA) B. subtilis PY79 recA-yfp::cat (RecA fused with yellow fluorescent protein [YFP]19).
- Harvest spores by centrifugation for 15 min at 3,000 x g in 50 mL tubes and purify the samples by repeated washing steps (up to 15 times) using sterile distilled H2O and check for purity and germination status by phase-contrast microscopy. Ensure that spore suspensions consist out of phase-bright spores (> 99%) and are free of vegetative cells (rods), germinated spores (black/ grey appearance) and cell debris, otherwise further microscopy experiments can be disturbed. Wash the sample until desired purity is reached.
- Determinate the spore titer by plating out 50 µL of 10-fold serial dilutions on LB-agar (i.e.: Use 30 µL sample + 270 µL sterile water for a 1:10 dilution. Take 30 µL from the particular dilution to 270 µL H2O for a 1:100 dilution and so forth) to calculate the CFU (colony forming units) and incubate the plates at 37 °C overnight. After CFU determination, adjust the sample to 109 spores per mL by concentrating or diluting with sterile water.
2. Sample Preparation of Aerosol-deposited Bacillus subtilis Spores
NOTE: Accumulation and overlapping of spores might lead to shadowing effects during the treatment, ultimately resulting in falsified inactivation kinetics. To minimize this problem, prepare spore samples by an aerosol-deposition technique20. Briefly, control the high-precision two-substance nozzle with an electric timer that regulates the liquid throughput in concert with the flow of pressurized carrier gas (here N2). Disperse the injected liquid sample through the nozzle outlet using the nitrogen gas flow.
- Place a sample carrier in form of sterilized microscopic slides (for survival kinetics) or round 25 mm coverslips (for fluorescent tracking of DNA repair processes/cLSM; confocal laser scanning microscopy) inside the electrically operated aerosol spraying unit in alignment with the nozzle. The used spore concentration needs to correspond to a hundredfold of the desired final concentration.
- Transfer 1 mL of the spore culture to the nozzle fluid inlet and initiate the spraying process of 0.1 s at a pressure of 1.3 bar. The sprayed spore suspension (1 x 107) forms a thin film on the microscopic slide that dries rapidly within seconds to form a uniformly distributed spore monolayer. Store the treated sample carriers in a sterile container at room temperature.
3. Low Pressure Plasma Treatment
- Prepare the plasma system for treatment of biological samples and operate the system at 5 Pa with argon plasma at 500 W for 5 min. By this, all surfaces in the system are cleaned and warmed up. This reduces sticking of molecules from ambient air, namely nitrogen, oxygen, and water, while venting the system. After the pretreatment of the system, vent the chamber and place the samples carefully in the center of the reactor vessel with the help of glass racks.
- Use at least three biological replicates. Close the chamber and evacuate below 2 Pa. Afterwards, fill the process gas into the chamber. Regulate the pressure in the system to 5 Pa.
- After the defined process time, turn off the power and gas supply, and carefully vent the system to prevent blowing the samples from the sample holder. After ventilation, remove the samples and place the samples for the next parameter in the system. For non-plasma-treated controls expose samples to vacuum only (5 Pa) in presence of the process gas equivalent to the longest applied plasma time.
4. Recovery and Evaluation of Spore Survival
- Prepare a solution of autoclaved 10% polyvinyl acetate (PVA) and cover the sample carrier carefully with approximately 500 µL and let them air-dry for 4 h. Strip off the dried PVA layer (now containing the spore sample) using sterile forceps and transfer it to a 2 mL reaction tube. Add 1 mL of sterile water to the tube and dissolve the PVA layer via vortexing. This procedure leads to >95% recovery of spores and does not affect their germination capability21.
- Serially dilute the sample at 1:10 in sterile water in a 96-well plate (i.e. 270 µL sterile H2O + 30 µL sample/former dilution). Plate out 50 µL of each dilution on Lysogeny broth nutrient agar (LB), incubate the plates at 37 °C overnight and enumerate the number of grown colonies (CFU).
5. Live Cell Microscopy and Tracking of DNA Repair Processes in Germinating Spores
- For germination experiments, prepare a 1 mm thick 1.5% LB-agar pad, by boiling 700 µL medium and pipet it into a sterile microscopy petri dish. After 10 min, cut out an 8 mm x 8 mm x 1 mm LB-agar pad with a sterile scalpel and transfer the agar carefully on top of the spore monolayers which are resting on 25 mm glass coverslips.
NOTE: This step is crucial for visualization of individual spores and allow following their reaction, towards the activation of germination induced by the nutrient agar. Thus, the LB-agar serves two purposes, (1) to fix the spores at the surface, which avoids relocalization along the surface and out of optical focus, and (2) to activate the spore for germination. - After covering the sample with agar, transfer the glass coverslip quickly into an imaging chamber and microscope the samples with an automated confocal laser-scanning microscope with inverted optics using a 63X/1.3 plane apochromatic oil immersion objective.
- Perform imaging of fluorescence (YFP) with an excitation wavelength of 514 nm and emission can be detected between 520 and 560 nm.
- Record bright-field images in scanning mode using one of the photo multipliers (transmitted-light path).
- Record time-lapse series with a laser power of 2.6%, and set the confocal aperture to 5 airy units and at a sample frequency of 1 frame per 30 s from 0 h to 5 h, depending on the experiment. It is of particular note that high doses of monochromatic laser illumination at 514 nm completely inhibit germination (Figure 1A, B).
- Keep the samples at 37 °C (ambient air humidity) in a heating stage during the entire imaging process. Use at least three biological replicates for each condition. In case of spore aggregation, multilayered spore distribution or contamination by dust particles, blocking of the plasma treatment ("shadowing") might occur and enable germination of shadowed spores (Figure 1C, D).
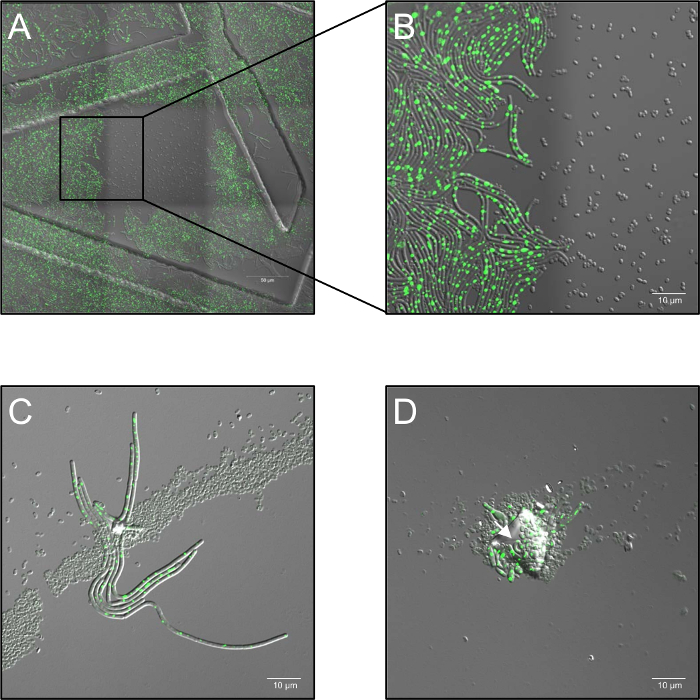
Figure 1: Potential problems observed during confocal live cell fluorescence microscopy of plasma treated spores. (A, B) Inhibition of spore germination by high doses of monochromatic (514 nm) laser illumination. (A) Overview (3 x 3 stitched frames) of B. subtilis (LAS72, RecA-YFP) spores 180 min after initiation of germination. The frame in the middle was exposed at 30 s intervals to high doses of laser light (514 nm, 70% laser power), whereas the surrounding regions (= frames) were not illuminated (merged image of bright-field channel and RecA-YFP fluorescence; ordered structures were caused by using 35 mm imaging dishes with an imprinted 500 µm grid). (B) demonstrates a 4X magnified view of the border between illuminated and non-illuminated region showing that spores, which were exposed to high doses of monochromatic laser illumination did not germinate and grow out, whereas spores in non-illuminated regions fully recover to vegetative bacteria expressing bright RecA-YFP fluorescence (green signal). (C, D) Spores covered by contaminating particles or multiple layers of spore (arrows) seems to protect underlying spores from inactivation by plasma treatment and allow their germination and outgrowth ("shadowing effect"). (C) Spores were plasma-treated for 60 s and imaged 180 min after initiation of germination or in (D) for 120 s and imaged after 240 min. Please click here to view a larger version of this figure.
6. Scanning Electron Microscopy (SEM)
- Use scanning electron microscopy to provide ultrastructural information about the surface morphology of plasma treated spores in comparison to untreated controls. Coat dried spore monolayers on coverslips with gold-palladium (3 nm) by using a sputter-coater. Use a field-emission scanning electron microscope for imaging the samples, operated at 5 kV acceleration voltage including an in-lens secondary electron detector to reveal topography contrast.
7. Data Analysis
- Determine the spore survival from the quotient N/N0, where N is the average CFU of treated samples and N0 is the average CFU of untreated vacuum controls. Plot spore inactivation by argon plasma treatment as a function of time (in seconds). Express all data as averages and standard deviations (n = 3).
- Analyze images obtained by live cell imaging using the imaging software. Quantify the proportion of spore germination and outgrowing after plasma treatment, count spores in representative frames at the beginning of the experiment as well as after 4 h. For significance determination in spore survival assays, use one-way ANOVA-tests (analysis of variance) with statistical software). P values <0.05 are considered as statistically significant.
Results
Survival of plasma-treated B. subtilis spores
Plasma treatment of the B. subtilis spores used in this study show a decrease in survival with increasing duration of the plasma treatment (Figure 2). Spores of the strain expressing the recA-gene fused to YFP showed survival curves similar to spores of the wild type strain, indicating that the genetic modification has no signific...
Discussion
Sterilization of surfaces using low-temperature, low-pressure plasma is a promising alternative to rather conventional sterilization procedures such as treatment with ionizing radiation, chemicals (e.g. gases like H2O2 or ethylene oxide) or dry and moist heat23. Ordinary sterilization methods mostly provide an effective sterilization, but they are known to influence the treated material and represent a potential risk for the operator. Low-pressure plasma offers a rap...
Disclosures
No conflicts of interest declared.
Acknowledgements
The authors thank Andrea Schröder for her excellent technical assistance during parts of this work and Nikea J. Ulrich for her assistance during the video shoot. We would also like to thank Lyle A. Simmons for his generous donation of the Bacillus subtilis strains: LAS72 and LAS24. This work was supported in parts by grants from the German Research Foundation (DFG) Paketantrag (PlasmaDecon PAK 728) to PA (AW 7/3-1) and RM (MO 2023/2-1) and the DLR grant DLR-FuW-Projekt ISS LIFE, Programm RF-FuW, Teilprogramm 475 (to F.M.F, M.R. and R.M.). F.M.F. was supported by a PhD scholarship of the Helmholtz Space Life Sciences Research School (SpaceLife) at the German Aerospace Center (DLR) in Cologne, Germany, which was funded by the Helmholtz Association (Helmholtz-Gemeinschaft) over a period of six years (Grant No. VH-KO-300) and received additional funds from the DLR, including the Aerospace Executive Board and the Institute of Aerospace Medicine. The results of this study will be included in the Ph.D. thesis of Felix M. Fuchs.
Materials
| Name | Company | Catalog Number | Comments |
| Two substance nozzle (model 970-8) | Schlick | 14,404 | 230 V, 50 Hz, D 4.484/8, 0.8 mm bore diameter |
| Luria Bertani Medium | Sigma Aldrich | 70122-100G | |
| Tube connectors | Festo | n/a | G 1/8 |
| Magnetvalve DO35-3/2NC-G018-230AC | Bosch Rexroth | 820005100 | |
| PLN Polyamid tube | Festo | 558206 | d = 6 mm |
| Glass slides | VWR | 48300-026 | |
| Electric Timer 550-2-C | Gefran | F000074 | 220 V |
| attofluor cell chamber | Menzel, Fisher Ref. | 3406816 | d=25 mm, round |
| MgSO4*7 H2O | Sigma Aldrich | 13152 | |
| Ca(NO3)2 | Sigma Aldrich | 202967 | |
| MnCl2 * 4 H2O | Sigma Aldrich | 244589 | |
| FeSO4 * 7H2O | AppliChem | 13446-34-9 | |
| Glucose | Merck | 215422 | |
| KCl | Sigma Aldrich | P9541-500G | |
| Nutrient Broth (NB) | Merck | 105443 | |
| Luria-Bertani (LB) | Merck | 110283 | |
| 96-wellplate | ThermoFisher | 243656 | |
| Zeiss LSM 780, Axio Observer Z1 | Carl Zeiss Microscopy GmbH | n/a | |
| Leo 1530 Gemini | Carl Zeiss Microscopy GmbH | n/a | |
| ZEN 2 and ZEN lite 2012 (Software) | Carl Zeiss Microscopy GmbH | n/a | |
| SigmaPlot, version 13.0 (Statistic software) | Systat GmbH, Erkrath, Germany | n/a | |
| Attofluor cell chamber | Invitrogen | A7816 | |
| µ-Dish 35 mm, high Grid-500 Glass Bottom | ibidi | 81168 |
References
- Nicholson, W. L., Schuerger, A. C., Race, M. S. Migrating microbes and planetary protection. Trends Microbiol. 17, 389-392 (2009).
- COSPAR. COSPAR Planetery Protection Policy. Space Research Today, COSPAR's Information Bulletin. 193, 1-14 (2015).
- De Geyter, N., Morent, R. Nonthermal plasma sterilization of living and nonliving surfaces. Annu Rev Biomed Eng. 14, 255-274 (2012).
- Shimizu, S., et al. Cold atmospheric plasma - A new technology for spacecraft component decontamination. Planet. Space Sci. 90, 60-71 (2014).
- Lerouge, S., Fozza, A. C., Wertheimer, M. R., Marchand, R., Yahia, L. H. Sterilization by Low-Pressure Plasma: The Role of Vacuum-Ultraviolet Radiation. Plasma Polym. 5, 31-46 (2000).
- Rossi, F., Kylián, O., Rauscher, H., Gilliland, D., Sirghi, L. Use of a low-pressure plasma discharge for the decontamination and sterilization of medical devices. Pure Appl. Chem. 80, 1939-1951 (2008).
- Halfmann, H., Hauser, J., Awakowicz, P., Koller, M., Esenwein, S. A. A double inductively coupled low-pressure plasma for sterilization of medical implant materials. Biomed Tech (Berl). 53, 199-203 (2008).
- Halfmann, H., Denis, B., Bibinov, N., Wunderlich, J., Awakowicz, P. Identification of the most efficient VUV/UV radiation for plasma based inactivation of Bacillus atrophaeus spores. J. Phys. D: Appl. Phys. 40, 5907 (2007).
- Vaishampayan, P., et al. Bacillus horneckiae sp. nov., isolated from a spacecraft-assembly clean room. Int J Syst Evol Microbiol. 60, 1031-1037 (2010).
- Mandic-Mulec, I., Stefanic, P., van Elsas, J. D. Ecology of Bacillaceae. Microbiol Spectr. 3, (2015).
- Alekhova, T. A., et al. Diversity of bacteria of the genus Bacillus on board of international space station. Dokl Biochem Biophys. 465, 347-350 (2015).
- Claus, D., Bekerley, R. C. W., Sneath, P. A. Genus Bacillus Cohn 1872. Bergey's manual of systematic bacteriology. 2, 1105-1141 (1986).
- Setlow, P. Spore Resistance Properties. Microbiol Spectr. 2, (2014).
- Setlow, P. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol. 101, 514-525 (2006).
- Roth, S., Feichtinger, J., Hertel, C. Characterization of Bacillus subtilis spore inactivation in low-pressure, low-temperature gas plasma sterilization processes. J Appl Microbiol. 108, 521-531 (2010).
- Roth, S., Feichtinger, J., Hertel, C. Response of Deinococcus radiodurans to low-pressure low-temperature plasma sterilization processes. J Appl Microbiol. 109, 1521-1530 (2010).
- Setlow, B., Setlow, P. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol. 178, 3486-3495 (1996).
- Schaeffer, P., Millet, J., Aubert, J. P. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. 54, 704-711 (1965).
- Simmons, L. A., et al. Comparison of responses to double-strand breaks between Escherichia coli and Bacillus subtilis reveals different requirements for SOS induction. J Bacteriol. 191, 1152-1161 (2009).
- Raguse, M., et al. Improvement of Biological Indicators by Uniformly Distributing Bacillus subtilis Spores in Monolayers To Evaluate Enhanced Spore Decontamination Technologies. Appl Environ Microbiol. 82, 2031-2038 (2016).
- Horneck, G., et al. Protection of bacterial spores in space, a contribution to the discussion on Panspermia. Orig Life Evol Biosph. 31, 527-547 (2001).
- Opretzka, J., Benedikt, J., Awakowicz, P., Wunderlich, J., Keudell, A. v. The role of chemical sputtering during plasma sterilization of Bacillus atrophaeus. J. Phys. D: Appl. Phys. 40, 2826 (2007).
- Stapelmann, K., et al. Utilization of low-pressure plasma to inactivate bacterial spores on stainless steel screws. Int. J. Astrobiol. 13, 597-606 (2013).
- Raguse, M., et al. Understanding of the importance of the spore coat structure and pigmentation in the Bacillus subtilis spore resistance to low-pressure plasma sterilization. J. Phys. D: Appl. Phys. 49, 285401 (2016).
- Pandey, R., et al. Live cell imaging of germination and outgrowth of individual Bacillus subtilis spores; the effect of heat stress quantitatively analyzed with SporeTracker. PloS one. 8, e58972 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved