A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Fabrication and Testing of Catalytic Aerogels Prepared Via Rapid Supercritical Extraction
In This Article
Summary
Here we present protocols for preparing and testing catalytic aerogels by incorporating metal species into silica and alumina aerogel platforms. Methods for preparing materials using copper salts and copper-containing nanoparticles are featured. Catalytic testing protocols demonstrate the effectiveness of these aerogels for three-way catalysis applications.
Abstract
Protocols for preparing and testing catalytic aerogels by incorporating metal species into silica and alumina aerogel platforms are presented. Three preparation methods are described: (a) the incorporation of metal salts into silica or alumina wet gels using an impregnation method; (b) the incorporation of metal salts into alumina wet gels using a co-precursor method; and (c) the addition of metal nanoparticles directly into a silica aerogel precursor mixture. The methods utilize a hydraulic hot press, which allows for rapid (<6 h) supercritical extraction and results in aerogels of low density (0.10 g/mL) and high surface area (200-800 m2/g). While the work presented here focuses on the use of copper salts and copper nanoparticles, the approach can be implemented using other metal salts and nanoparticles. A protocol for testing the three-way catalytic ability of these aerogels for automotive pollution mitigation is also presented. This technique uses custom-built equipment, the Union Catalytic Testbed (UCAT), in which a simulated exhaust mixture is passed over an aerogel sample at a controlled temperature and flow rate. The system is capable of measuring the ability of the catalytic aerogels, under both oxidizing and reducing conditions, to convert CO, NO and unburned hydrocarbons (HCs) to less harmful species (CO2, H2O and N2). Example catalytic results are presented for the aerogels described.
Introduction
Silica- and alumina-based aerogels have remarkable properties, including low density, high porosity, high surface area, good thermal stability and low thermal conductivity1. These properties render the aerogel materials attractive for a variety of applications1,2. One application that exploits the thermal stability and high surface area of aerogels is heterogeneous catalysis; several articles review the literature in this area2,3,4,5. There are many approaches to the fabrication of aerogel-based catalysts, including incorporation or entrapment of catalytic species within the framework of a silica or alumina aerogel5,6,7,8,9,10,11. The present work focuses on protocols for preparation via rapid supercritical extraction (RSCE) and catalytic testing of aerogel materials for automotive pollution mitigation, and uses copper-containing aerogels as examples.
Three-way catalysts (TWCs) are commonly employed in pollution mitigation equipment for gasoline engines12. Modern TWCs contain platinum, palladium and/or rhodium, platinum-group metals (PGMs) that are rare and, therefore, expensive and environmentally costly to obtain. Catalyst materials based on more readily available metals would have significant economic and environmental advantages.
Aerogels can be prepared from wet gels using a variety of methods1. The goal is to avoid pore collapse as solvent is removed from the gel. The process employed in this protocol is a rapid supercritical extraction (RSCE) method in which the extraction occurs from a gel confined within a metal mold in a programmable hydraulic hot press13,14,15,16. The use of this RSCE process for the fabrication of silica aerogel monoliths has been previously demonstrated in a protocol17, in which the relatively short preparation time associated with this approach was emphasized. Supercritical CO2 extraction is a more common approach, but takes more time and requires greater use of solvents (including CO2) than RSCE. Other groups have recently published protocols for preparation of a variety of types of aerogels utilizing supercritical CO2 extraction18,19,20.
Here, protocols for fabricating and catalytically testing a variety of types of copper-containing catalytic aerogels are presented. Based on the NO reduction and CO oxidation activity ranking of carbon-supported base metal catalysts under conditions of interest to automotive pollution mitigation provided by Kapteijn et al.21, copper was selected as the catalytic metal for this work. Fabrication approaches include (a) impregnation (IMP) of copper salts into alumina or silica wet gels11, (b) using copper(II) and aluminum salts as co-precursors (Co-P) when fabricating copper-alumina aerogels6,22, and (c) entrapping copper-containing nanoparticles into a silica aerogel matrix during fabrication10. In each case, an RSCE method is used for removal of solvent from the pores of the wet gel matrix13,14,15.
A protocol for assessment of the suitability of these materials as TWCs for automotive pollution mitigation, using the Union Catalytic Testbed (UCAT)23, is also presented. The purpose of the UCAT system, key portions of which are shown schematically in Figure 1, is to simulate the chemical, thermal, and flow conditions experienced in a typical gasoline engine catalytic converter. UCAT functions by passing a simulated exhaust mixture over an aerogel sample at a controlled temperature and flow rate. The aerogel sample is loaded into a 2.25-cm-diameter tubular packed bed flow cell ("test section"), which contains the sample between two screens. The loaded flow cell is placed into an oven to control the exhaust gas and catalyst temperature, and samples of treated exhaust (i.e. exhaust flowed through the packed bed) and untreated gas (i.e. bypassing the packed bed) are examined at a range of temperatures up to 700 ˚C. The concentrations of the three key pollutants -- CO, NO, and unburned hydrocarbons (HCs) -- are measured using a five-gas analyzer after being treated by the aerogel catalyst and, separately, in an untreated ("bypass") flow; from these data the "percent conversion" for each pollutant is calculated. For the testing described herein, a commercially available exhaust blend, California Bureau of Automotive Repair (BAR) 97 LOW emissions blend was employed. Full details of the UCAT's design and functioning are presented in Bruno et al.23
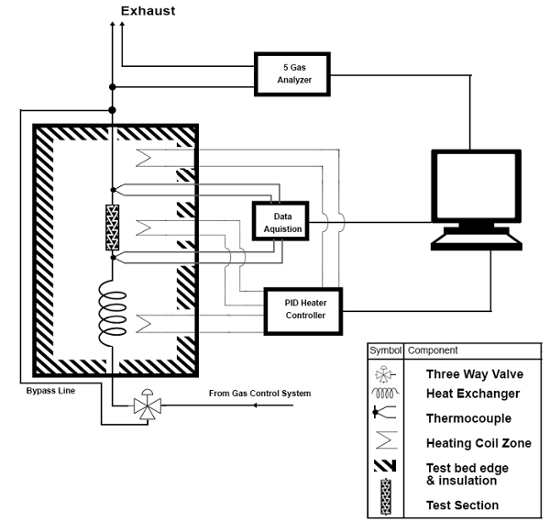
Figure 1. UCAT Test Section and Sampling Systems. Reprinted with permission from 2016-01-0920 (Bruno et al.23), Copyright 2016 SAE International. Further distribution of this material is not permitted without prior permission from SAE. Please click here to view a larger version of this figure.
Protocol
Safety Considerations: Wear safety glasses or goggles and laboratory gloves at all times when performing preparatory work with chemical solutions and when handling wet gels or catalytic aerogel materials. Handle propylene oxide, tetramethyl orthosilicate (TMOS), ethanol, methanol, ammonia, nanoparticles and solutions containing any of these within a fume hood. Read Safety Data Sheets (SDS) for all chemicals, including nanoparticles, prior to working with them. Wear a particulate mask when crushing aerogel samples and during loading and unloading of the test cell. Wear safety glasses or goggles when operating the hydraulic hot press or catalytic test bed. Tie back long hair and do not wear loose clothing (scarves, for example) when working with the hot press. As noted in our previous protocol17, employ a safety shield around the hot press, properly vent the hot press and make certain that there are no ignition sources nearby. Provide correct ventilation of the test bed and all gas exhausts and. Install NO and CO gas monitors in the operator space associated with the catalytic test bed. Wear oven gloves when removing or replacing a hot test cell.
1. Fabrication of Alumina-Copper Sol Gels using Copper Salts
Note: Recipes for alumina-copper (Al-Cu) sol gels are shown in Table 1. All solution preparations are performed within a fume hood.
- Prepare Reagents and Other Supplies
- Gather reagents needed: aluminum chloride hexahydrate, copper nitrate trihydrate, propylene oxide, reagent-grade ethanol, and absolute ethanol.
- Obtain supplies needed: clean and dry beakers (two 250-mL); clean, dry, magnetic stir bar; 50- or 100-mL graduated cylinder; one 10-mL hypodermic syringe; one calibrated digital balance.
- Obtain a small laboratory-scale sonicator and prepare for use by adding water to the fill line and ensuring that both beakers can be placed in sonicator without tipping over.
- Synthesize Alumina-Copper Sol Gels via an Impregnation Method (Al-Cu IMP)
- Using a calibrated digital balance, weigh out 5.92 g of aluminum chloride hexahydrate, and add to the 250-mL beaker. Add 40 mL of reagent-grade ethanol and a stir bar to the same 250-mL beaker. Cover the beaker with paraffin film and place on magnetic plate for stirring at moderate speed until the aluminum salt has dissolved (approximately 15 min). Remove beaker from magnetic plate and uncover.
- Use the 10-mL syringe to pierce the septum on the propylene oxide bottle and add 8 mL of propylene oxide to the 250-mL beaker. Replace paraffin film on the beaker and place on the magnetic plate for stirring at moderate speed until the solution has gelled (approximately 5 min). Remove the beaker from the magnetic plate and allow gel to age at room temperature for 24 h.
- Using a calibrated digital balance, weigh out 1.4 g of copper nitrate trihydrate and add to a beaker. Add 40 mL of absolute ethanol to the beaker. Place the beaker in the sonicator and sonicate until the copper salt dissolves (approximately 10 min).
- Pour any excess solvent off alumina gel, remove the stir bar and break the gel into several pieces (5-10 mm per side) using a spatula. Pour copper solution into the beaker containing the gel. Cover beaker with paraffin film, and allow the gel to age at room temperature for 24 h.
- Pour off excess solvent and add 40 mL of fresh absolute ethanol. Replace paraffin film on the beaker, and allow the gel to age for another 24 h at room temperature.
- Repeat step 1.2.5 at least once to ensure removal of excess propylene oxide (reagent) and any reaction byproducts6.
- Proceed to Step 3 (Processing...into Aerogels...) to perform supercritical extraction of solvent from wet gels to yield aerogels.
- Synthesize Alumina-Copper Sol Gels via a Co-Precursor Method (Al-Cu CoP)
- Using a calibrated digital balance, weigh out 4.52 g of aluminum chloride hexahydrate, and 1.4 g of copper nitrate trihydrate. Add these salts to a clean 250-mL beaker. Add 40 mL of reagent-grade ethanol and a stir bar to the 250-mL beaker. Cover the beaker with paraffin film and place on magnetic plate for stirring at moderate speed until the aluminum and copper salts have dissolved (approximately 15 min). Remove the beaker from magnetic plate and uncover.
- Use the 10-mL syringe to pierce the septum on the propylene oxide bottle, and add 9.5 mL propylene oxide to the 250-mL beaker. Replace paraffin film on the beaker and place on magnetic plate. Stir until the solution has gelled (15-20 min). Remove the beaker from the magnetic plate and allow the gel to age at room temperature for 24 h.
- Pour any excess solvent off gel, and break the gel into several pieces (5-10 mm per side) using a spatula. Add 40 mL of fresh absolute ethanol to beaker, cover 250-mL beaker with paraffin film, and allow the gel to age at room temperature for 24 h.
- Pour off excess solvent and add 40 mL of fresh absolute ethanol. Replace paraffin film on beaker and allow the gel to age for another 24 h at room temperature.
- Repeat step 1.3.4. at least once in order to remove excess propylene oxide and any reaction byproducts.
- Proceed to Step 3 (Processing...into Aerogels...) to perform supercritical extraction of solvent from wet gels to yield aerogels.
2. Fabrication of Silica-Copper Sol Gels using Copper Salts
Note: The recipe for silica-copper (Si-Cu) sol gels is shown in Table 2. All solution preparations are performed within a fume hood.
- Prepare Reagents and Other Supplies
- Gather reagents needed: tetramethyl orthosilicate (TMOS), methanol, deionized water, ammonia, copper nitrate trihydrate, and absolute ethanol.
- Make 100 mL of a 1.5-M ammonia solution by diluting 10.1 mL of 14.8-M concentrated ammonia to 100 mL with deionized water.
- Obtain supplies needed: clean and dry beakers (including one 250-mL and one 100-mL beaker); calibrated variable-volume pipettes (one 1000- µL and one 10.0-mL digital pipette with appropriate tips are recommended); one 50-mL or 100-mL graduated cylinder; one calibrated digital balance.
- Obtain small laboratory-scale sonicator and prepare for use by adding water to the fill line and ensuring that both beakers can be placed in sonicator without tipping over.
- Synthesize Silica-Copper Sol Gel via an Impregnation Method (Si-Cu IMP)
- Pipette 8.5 mL of TMOS into the 250-mL beaker. Add 27.5 mL of methanol to the 250-mL beaker using a graduated cylinder. Pipette 3.6 mL of water into the 250-mL beaker. Cover 250-mL beaker with paraffin film and sonicate the mixture until it is a monophasic solution (5-10 min), then uncover.
- Pipette 1.35 mL of 1.5-M NH3 into the 250-mL beaker. Replace paraffin film on the beaker and sonicate until gelation occurs (approximately 2 min). Allow the gel to age at room temperature for 24 h.
- Using a calibrated digital balance, weigh out 0.55 g of copper nitrate trihydrate and add to a 100-mL beaker. Add 20 mL of absolute ethanol to 100-mL beaker. Place 100-mL beaker in the sonicator and sonicate until copper salt has fully dissolved (approximately 10 min).
- Break the silica gel into several pieces (5-10 mm per side) using a spatula, and add copper solution to the 250-mL beaker containing the gel. Replace paraffin film on beaker and allow the gel to age at room temperature for 24 h.
- Pour off excess solvent and add 20 mL of fresh absolute ethanol. Replace paraffin film on beaker and allow gel to age for another 24 h.
- Repeat step 2.2.5. at least once.
- Proceed to Step 3 (Processing...into Aerogels...) to perform supercritical extraction of solvent from wet gels to yield aerogels.
3. Processing Alumina-Copper and Silica-Copper Sol Gels made using Copper Salts into Aerogels via Rapid Supercritical Extraction
- Prepare Hot Press and Mold
- Obtain an appropriately sized stainless-steel mold. For example, a 12.7 cm x 12.7 cm x 1.8 cm mold with four circular wells measuring 3.8 cm in diameter and 1.5 cm in depth.
- Prepare gasket material. Cut sealing gaskets sufficient in size to cover the mold fully (in this example, >12.7 cm x >12.7 cm) from 1.6-mm-thick graphite gasket material and 0.012-mm thick stainless-steel foil.
- Program the hot press for ethanol extraction, see Table 3 for parameters.
- Perform Supercritical Extraction in Hot Press
- Following preparation and ethanol exchange of wet gels (step 1.2.6, 1.3.5 or 2.2.6), decant excess solvent.
- Distribute the wet sol gels into the wells of the mold, and center the mold on the hot press heating plate. Top off each well with absolute ethanol.
- Place gasket materials, used to seal the mold, on top of the mold: stainless steel foil first, then the graphite sheet.
- Begin the hot press extraction program.
- Once the process is complete (approximately 5 h), remove mold from hot press. Remove gasket material from the mold, and transfer aerogels into sample containers.
4. Fabrication of Copper-Nanoparticle-Doped Silica Aerogel Monoliths (Si-Cu NP)
- Prepare Reagents and Supplies
- Gather reagents: TMOS, methanol, deionized water, 25- to 55-nm sized copper (II) oxide nanoparticles dispersed in water at 20 wt%, and 1.5-M aqueous ammonia solution (as described in step 2.1.2.). Different types (oxidation states, sizes) and concentration of nanoparticles can be used with adjustments to the recipe.
- Prepare supplies: clean and dry beakers (including one 250 mL and one 100 mL); calibrated variable-volume pipettes (one 10-mL and one 1,000- µL digital pipette with appropriate tips are recommended); disposable Pasteur pipette; one calibrated digital balance.
- Obtain small laboratory-scale sonicator and prepare for use by adding water to the fill line and ensuring that both beakers can be placed in sonicator without tipping over.
- Prepare Hot Press and Mold
- Prepare appropriately sized steel mold. In this example, a 12.7 cm x 12.7 cm x 1.905-cm mold, with nine circular through wells of 1.905-cm diameter. Spray wells with high temperature lubricant to facilitate removal of aerogels after processing.
- Prepare gasket material. Gather 1.6-mm-thick graphite gasket material and 0.012-mm-thick stainless-steel foil and cut three pieces of each sufficient in size to cover the mold fully (in this example, >12.7 cm x >12.7 cm).
- Program hot press for sealing and extraction. Refer to Table 4 and Table 5, respectively, for program values.
Note: Sealing is necessary to prevent liquid from seeping out of the mold 's open-bottom wells. - Place gasket material and the mold in the center of the hot press platens in the following order: graphite, foil, mold, foil, graphite. Start the sealing program (using parameters in Table 4).
- Make Precursor Solution for Si-Cu NP Aerogels
Note: The recipe for the silica aerogel containing 5 wt% copper (II) oxide nanoparticles is listed in Table 6. This recipe can be modified to incorporate different weight percentage amounts of copper. All solutions should be handled and mixed in a fume hood.- Place a clean 250-mL beaker on the calibrated digital balance and pipette approximately 13 mL of TMOS into the 250-mL beaker. Add additional TMOS as needed with the Pasteur pipette for a total of 13.04 g of TMOS.
- Pipette a total of 32.63 g methanol into the 250-mL beaker. Pipette 3.90 g deionized water into the 250-mL beaker.
- Shake the 20 wt% copper (II) oxide nanodispersion to ensure any nanoparticles that have settled to the bottom are re-suspended, then pipette 1.50 g of the nanodispersion into the 250-mL beaker of precursor solution. Pipette 200 µL of 1.5-M ammonia into the 250-mL beaker.
- Cover the beaker with paraffin film and sonicate the mixture for 5-10 min until it is a monophasic solution.
- Perform Gelation and Supercritical Extraction in Hot Press
- After the sealing program is complete, remove the top gasket, taking care not to move the mold. At this point, the bottom of the mold has been sealed.
- Fill each well completely with the precursor solution.
Note: There will be solution left over. This can be discarded or left to dry under ambient conditions to make xerogels. - Place a fresh piece of foil then a fresh piece of graphite on top of the mold.
- Start extraction program (using parameters in Table 5).
- When the extraction program is complete (approximately 8 h), remove the mold and gasket material from the hot press. Gently peel the gasket material from the top of the mold and discard it. Carefully push each aerogel into a sample container using a gloved finger.
5. Operating the Union Catalytic Test Bed
- Prepare and Load Sample
- Lightly crush approximately 30 mL of aerogel into approximately 1- to 2-mm diameter pieces using a mortar and pestle. Do not crush aerogel into a powder.
- Measure approximately 30 mL of catalytic aerogel pieces using a clean, dry graduated cylinder.
Note: The aerogels will shrink with heat treatment, so it is necessary to ensure that there is 15 - 20 mL of aerogel available to test after heat treatment. - Place the aerogel into ceramic crucibles, cover the crucibles loosely, and calcine in a furnace at 800 ˚C for 24 h.
- Remove crucibles from furnace and let cool.
- Measure 20 mL of aerogel and pour into a clean, dry UCAT test section and insert an end screen to retain the sample in place during testing.
- Load test section into the UCAT assembly using copper washers and clamps to seal. Securely close the UCAT oven.
Note: To avoid oven damage or electrical short circuits, ensure that test section does not contact the interior wall of oven.
- Prepare the Union Catalytic Test Bed
- Check that CO and NO detectors are on and functioning.
- Check simulated exhaust gas supply. Replace simulated exhaust bottle before beginning test if pressure is below 700 kPa.
- Set the gas pressure regulator to 345 kPa. Set air pressure regulator to 345 kPa. Leak test exhaust gas flow lines.
- Turn on and zero the calibrated five-gas analyzers. Set analyzers to measure. Leave analyzers on for 30 min to warm up.
- Set the desired oven temperature (typically 200 ˚C for first reading) and start the oven. Ensure that the bypass valve is set to deliver air through the test cell.
- Adjust mass flow rate controllers to deliver the correct quantities of air (used during warmup) and simulated exhaust (used during testing) to maintain desired space velocity.
Note: In our system this is accomplished simply by setting the desired space velocity in the system 's control program. The mass flow controllers are automatic and adjust the mass flowrates to the required values, based on the oven temperature, to maintain the selected space velocity. - Turn on the warm up / purge air flow through the test cell and wait for the flow through the test cell to stabilize at desired test temperature (typically 30 min).
- Take a measurement.
- Re-zero the five-gas analyzer and set the bypass valve to send flow to bypass the test section. Turn off the warm up / purge air.
- Turn on the simulated exhaust flow. Allow five-gas analyzer readings to stabilize (approx. 90 s) and record bypass (simulated exhaust bottle) pollutant concentrations.
- Set the bypass valve to direct the flow through the test section. Allow five gas analyzer readings to stabilize (approx. 360 s) and record treated no-oxygen exhaust pollutant concentrations.
- Turn on oxygen addition to the blend. Allow five-gas analyzer readings to stabilize (approx. 90 s) and record treated with-oxygen exhaust pollutant concentrations.
- Set the bypass valve to send flow to bypass the test section. Allow five-gas analyzer readings to stabilize (approx. 90 s) and record bypass (simulated exhaust bottle) pollutant concentrations again.
- Turn off the simulated exhaust flow.
- Increment oven temperature to next desired condition (typically 50 ˚C higher), then repeat steps 5.2.6 to 5.3.6. Continue until measurements have been completed at the desired maximum temperature (typically 600 ˚C).
- Closing the Union Catalytic Test Bed
Note: After completing the final bypass (typ at 600 ˚C) the test is complete. Shut down the test bed.- Turn off simulated exhaust bottle valves and regulators. Turn off oven, five-gas analyzer and air.
| Chemical | Amount (Impregnation Method) | Amount (Co-Precursor Method) |
| AlCl3•7H2O | 5.92 g | 4.52 g |
| Cu(NO3)2•3H2O | 1.4 g | 1.4 g |
| Propylene oxide | 8 mL | 9.5 mL |
| Reagent-grade ethanol | 40 mL | 40 mL |
| Absolute ethanol | 120 mL | 120 mL |
Table 1. Recipe for Preparation of Alumina-Copper Sol Gels.
| Chemical | Amount (Impregnation Method) |
| TMOS | 8.5 mL |
| MeOH | 27.5 mL |
| H2O | 3.6 mL |
| 1.5-M NH3 | 1.35 mL |
| Absolute Ethanol | 60 mL |
| Cu(NO3)2•3H2O | 0.55 g |
Table 2. Recipe for Preparation of Silica-Copper Sol Gels.
| Step # | Temperature (°C) | Temp Rate (°C/min) | Force (kN) | Force Rate (kN/min) | Dwell Time (min) |
| 1 | 30 | 300 | 200 | 3000 | 0.25 |
| 2 | 250 | 2.2 | 200 | -- | 30 |
| 3 | 250 | -- | 4.5 | 4.5 | 15 |
| 4 | 30 | 2.2 | 4.5 | -- | 1 |
| 5 | END |
Table 3. Hot-Press Extraction Program Parameters for Alumina-Copper and Silica-Copper Sol Gels.
| Step # | Temperature (°C) | Temp Rate (°C/min) | Force (kN) | Force Rate (kN/min) | Dwell Time (min) |
| 1 | OFF | -- | 90 | 3000 | 10 |
| 2 | END |
Table 4. Hot-Press Sealing Program Parameters.
| Step # | Temperature (°C) | Temp Rate (°C/min) | Force (kN) | Force Rate (kN/min) | Dwell Time (min) |
| 1 | 30 | 300 | 180 | 3000 | 0.25 |
| 2 | 290 | 1.6 | 180 | -- | 30 |
| 3 | 290 | -- | 4.5 | 4.5 | 15 |
| 4 | 40 | 1.6 | 4.5 | -- | 1 |
| 5 | END |
Table 5. Hot-Press Extraction Program Parameters for Copper-nanoparticle-doped Silica aerogels.
| Chemical | Amount (mL) | Amount (g) |
| TMOS | 12.75 | 13.04 |
| Methanol | 41.25 | 32.63 |
| Water | 3.9 | 3.9 |
| Nanodispersion | 1.5 | 1.5 |
| Ammonia | 0.2 | 0.15 |
Table 6. Recipe for Fabrication of 5 wt% Copper-nanoparticle-doped Silica Aerogels.
Results
Photographic images of the resulting aerogels are presented in Figure 2. Because the wet gels were broken into pieces prior to solvent exchange, the Al-Cu IMP and Si-Cu IMP aerogels are in small, irregularly shaped monolithic pieces. It is clear from the coloration of these samples that the aerogels contain copper species and that variations in copper speciation and/or ligand structure occur within the materials. Al-Cu IMP aerogels (Figur...
Discussion
The utility of the RSCE method for fabrication of catalytic aerogels and the UCAT system for demonstrating catalytic ability has been demonstrated herein. Major advantages of these protocols over other methods are the speed of RSCE aerogel fabrication and the relatively inexpensive approach to catalytic testing by UCAT.
Gels to be extracted can be prepared via a variety of methods, including impregnation of metal salts into an alumina or silica wet gel matrix, inclusion of metal salts as co-pr...
Disclosures
The authors have nothing to disclose.
Acknowledgements
Development of the synthesis methods for catalytic aerogels was funded through National Science Foundation (NSF) grant No. DMR-1206631. The design and construction of UCAT was funded through NSF grant No. CBET-1228851. Additional funding was provided by the Union College Faculty Research fund. The authors would also like to acknowledge the contributions of Zachary Tobin, Aude Bechu, Ryan Bouck, Adam Forti, and Vinicius Silva.
Materials
| Name | Company | Catalog Number | Comments |
| Variable micropipettor, 100-1000 µL | Manufactured by Eppendorf, purchased from Fisher Scientific www.fishersci.com | S304665 | Any 100-1000 µL pipettor is suitable. |
| Variable Pipettor, 2.5-10 mL | Manufactured by Eppendorf, purchased from Fisher Scientific www.fishersci.com | 21-379-25 | Any variable pipettor is suitable. |
| Pasteur pipettes | FisherScientific | 13-678-6A | |
| Syringe | Purchased from Fisher Scientific | Z181390 syringe with Z261297 needle | |
| Digital balance | OHaus Explorer Pro | Any digital balance is suitable. | |
| Beakers | Purchased from Fisher Scientific | Any glass beaker is suitable. | |
| Graduated Cylinder | Purchased from Fisher Scientific | Any glass graduated cylinder is suitable. | |
| Magnetic Plate/Stirrer | FisherScientific Isotemp | SP88854200P | Any magnetic plate/stirrer is suitable. |
| Ultrasonic Cleaner | FisherScientific FS6 | 153356 | Any sonicator is suitable. |
| Mold | Fabricated in House | Fabricate from cold-rolled steel or stainless steel. | |
| Hydraulic Hot Press | Tetrahedron www.tetrahedronassociates.com | MTP-14 | Any hot press with temperature and force control will work. Needs maximum temperature of ~550 F and maximum force of 24 tons. |
| UCAT (Union Catalytic Testbed) | Fabricated in House | Described in detail in reference #21: Bruno, B.A., Anderson, A.M., Carroll, M.K., Brockmann, P., Swanton, T., Ramphal, I.A., Palace, T. Benchtop Scale Testing of Aerogel Catalysts. SAE Technical Paper 2016-01-920 (2016). | |
| Bar 97 Gas | Praxair | MS_BAR97ZA-D7 |
References
- Aegerter, M. A., Leventis, N., Koebel, M. M. . Aerogels Handbook. , (2011).
- Pierre, A. C., Pajonk, G. M. Chemistry of Aerogels and Their Applications. Chem. Rev. 102 (11), 4243-4266 (2002).
- Schneider, M., Baiker, A. Aerogels in Catalysis. Catal. Rev. 37, 515-556 (1995).
- Vallribera, A., Molins, E., Astruc, D. Aerogel Supported Nanoparticles in Catalysis. Nanoparticles and Catalysis. , (2007).
- Amonette, J. E., Matyas, J. Functionalized silica aerogels for gas-phase purification, sensing, and catalysis: A review. Mircopor. Mesopor. Mater. 250, 100-119 (2017).
- Juhl, S. J., Dunn, N. J. H., Carroll, M. K., Anderson, A. M., Bruno, B. A., Madero, J. E., Bono, M. S. Epoxide-Assisted Alumina Aerogels by Rapid Supercritical Extraction. J. Non-Cryst. Solids. 426, 141-149 (2015).
- Bono, M. S., Dunn, N. J. H., Brown, L. B., Juhl, S. J., Anderson, A. M., Bruno, B. A., Mahony, M. K. Catalyst, Catalytic Converter and Method for the Production Thereof. US Patent. , (2016).
- Smith, L. C., Anderson, A. M., Carroll, M. K. Preparation of vanadia-containing aerogels by rapid supercritical extraction for applications in catalysis. J. Sol-Gel Sci. Technol. 77, 160-171 (2016).
- Bouck, R. M., Anderson, A. M., Prasad, C., Hagerman, M. E., Carroll, M. K. Cobalt-alumina Sol Gels: Effects of Heat Treatment on Structure and Catalytic Ability. J. Non-Cryst. Solids. 453, 94-102 (2016).
- Anderson, A. M., Donlon, E. A., Forti, A. A., Silva, V., Bruno, B. A., Carroll, M. K. Synthesis and Characterization of Copper-Nanoparticle-Containing Silica Aerogel Prepared Via Rapid Supercritical Extraction for Applications in Three-Way Catalysis. MRS Advances. , 1-6 (2017).
- Tobin, Z. M., Posada, L. F., Bechu, A. M., Carroll, M. K., Bouck, R. M., Anderson, A. M., Bruno, B. A. Preparation and Characterization of Copper-containing Alumina and Silica Aerogels for Catalytic Applications. J. Sol-Gel Sci. Technol. , (2017).
- Heck, R., Farrauto, R., Gulati, S. . Catalytic Air Pollution Technology. , (2009).
- Gauthier, B. M., Bakrania, S. D., Anderson, A. M., Carroll, M. K. A Fast Supercritical Extraction Technique for Aerogel Fabrication. J. Non-Cryst. Solids. 350, 238-243 (2004).
- Gauthier, B. M., Anderson, A. M., Bakrania, S. D., Mahony, M. K., Bucinell, R. B. Method and Device for Fabricating Aerogels and Aerogel Monoliths Obtained Thereby. US Patent No. , (2008).
- Gauthier, B. M., Anderson, A. M., Bakrania, S. D., Mahony, M. K., Bucinell, R. B. Method and Device for Fabricating Aerogels and Aerogel Monoliths Obtained Thereby. US Patent. , (2011).
- Roth, T. B., Anderson, A. M., Carroll, M. K. Analysis of a Rapid Supercritical Extraction Aerogel Fabrication Process: Prediction of Thermodynamic Conditions During Processing. J. Non-Cryst. Solids. 354 (31), 3685-3693 (2008).
- Carroll, M. K., Anderson, A. M., Gorka, C. A. Preparing Silica Aerogel Monoliths via a Rapid Supercritical Extraction Method. J. Vis. Exp. (84), e51421 (2014).
- Harper-Leatherman, A. S., Pacer, E. R., Kosciuszek, N. D. Encapsulating Cytochrome c in Silica Aerogel Nanoarchitectures without Metal Nanoparticles while Retaining Gas-phase Bioactivity. J. Vis. Exp. (109), e53802 (2016).
- Subrahmanyam, R., Gurikov, P., Meissner, I., Smirnova, I. Preparation of Biopolymer Aerogels Using Green Solvents. J. Vis. Exp. (113), e54116 (2016).
- Campbell, P. G., Worsley, M. A., Hiszpanski, A. M., Baumann, T. F., Biener, J. Synthesis and Functionalization of 3D Nano-graphene Materials: Graphene Aerogels and Graphene Macro Assemblies. J. Vis. Exp. (105), e53235 (2015).
- Kapteijn, F., Stegenga, S., Dekker, N. J. J., Bijsterbosch, J. W., Moulijn, J. A. Alternatives to noble metal catalysts for automotive exhaust purification. Catalysis Today. 16 (2), 273-287 (1993).
- Baumann, T., Gash, A., Chinn, S., Sawvel, A., Maxwell, R., Satcher, J. Synthesis of high-surface-area alumina aerogels without the use of alkoxide precursors. Chem. Mater. 17, 395-401 (2005).
- Bruno, B. A., Anderson, A. M., Carroll, M. K., Brockmann, P., Swanton, T., Ramphal, I. A., Palace, T. Benchtop Scale Testing of Aerogel Catalysts. SAE Technical Paper 2016-01-920. , (2016).
- Anderson, A. M., Wattley, C. W., Carroll, M. K. Silica Aerogels Prepared via Rapid Supercritical Extraction: Effect of Process Variables on Aerogel Properties. J. Non-Cryst. Solids. 355 (2), 101-108 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved