A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Experimental Protocol for Using Drosophila As an Invertebrate Model System for Toxicity Testing in the Laboratory
In This Article
Summary
In this paper, we provide a detailed protocol for exposing species in the genus Drosophila to pollutants with the goal of studying the impact of exposure on a range of phenotypic outputs at different developmental stages and for more than one generation.
Abstract
Emergent properties and external factors (population-level and ecosystem-level interactions, in particular) play important roles in mediating ecologically-important endpoints, though they are rarely considered in toxicological studies. D. melanogaster is emerging as a toxicology model for the behavioral, neurological, and genetic impacts of toxicants, to name a few. More importantly, species in the genus Drosophila can be utilized as a model system for an integrative framework approach to incorporate emergent properties and answer ecologically-relevant questions in toxicology research. The aim of this paper is to provide a protocol for exposing species in the genus Drosophila to pollutants to be used as a model system for a range of phenotypic outputs and ecologically-relevant questions. More specifically, this protocol can be used to 1) link multiple biological levels of organization and understand the impact of toxicants on both individual- and population-level fitness; 2) test the impact of toxicants at different stages of developmental exposure; 3) test multigenerational and evolutionary implications of pollutants; and 4) test multiple contaminants and stressors simultaneously.
Introduction
Every year, approximately 1,000 new chemicals are introduced by the chemical industry1,2; however, the environmental impacts of only a small percentage of these chemicals are tested before distribution2,3. Although large-scale catastrophes are uncommon, sublethal and chronic exposure to a large variety of pollutants are widespread in both humans and wildlife4,5. The historical focus of ecotoxicology and environmental toxicology was to test lethality, single chemical exposure, acute exposure, and the physiological effects of exposure, as a means of measuring the impact of pollutants on survival6,7,8,9,10. Although there is a shift towards ethical and non-invasive approaches to animal testing, current approaches are limiting because of the role that development, emergent properties, and external factors (such as population-level and ecosystem-level interactions) play in mediating ecologically-important endpoints8. Therefore, there is a need for methods that incorporate a more holistic approach without sacrificing wildlife and/or vertebrates in the laboratory.
Invertebrate model systems, such as Drosophila melanogaster, are an attractive alternative to address the need for a more holistic approach to toxicity testing. D. melanogaster, was originally developed as an invertebrate model system for human-related genetic research about a century ago11.D. melanogaster is now prominently used as a vertebrate model alternative for several reasons: 1) the conservation of genes and pathways between D. melanogaster and humans; 2) short generation time compared to vertebrate models; 3) inexpensive cost of maintenance; 4) ease in generating large sample sizes; and 5) plethora of phenotypic- and ecologically-relevant endpoints available for testing11,12,13,14,15,16,17.
Several laboratories11,15,16,17,18,19,20,21,22,23,24,25 are now using D. melanogaster as a vertebrate model alternative for toxicity testing to understand the impacts of pollution on humans. Local wild species of Drosophila can be utilized, as well, as toxicity models for wildlife (and humans) to answer ecologically-, behaviorally-, and evolutionarily-relevant questions at multiple biological levels of organization. Using species within the Drosophila genus as a model, several measurable endpoints are possible11,15,16,18,19,20,21,22,23,24,25. In addition, using the Drosophila model, toxicologists can: 1) ethically link effects at multiple biological levels of the organization; 2) incorporate the role of emergent factors and development; 3) study ecologically-important endpoints (in addition to medically-important endpoints); 4) test multiple stressors simultaneously; 5) and test long-term multigenerational (e.g. evolutionary and transgenerational) implications of stressors. Therefore, using Drosophila as a model system enables a multitude of approaches, not limited to studying mechanistic approaches with inbred strains of D. melanogaster in the laboratory.
In this paper, we present the methods for rearing and collecting Drosophila to answer various toxicological questions. More specifically, we describe the methodology for 1) rearing Drosophila in medium laced with one or more pollutants; 2) collecting Drosophila throughout development (e.g. wandering third-instar larvae, pupal cases, newly-eclosed adults, and mature adults); and 3) rearing Drosophila in the contaminated medium to test intergenerational and transgenerational transmission, as well as evolutionary implications of long-term toxicant exposure. Using this protocol, previous authors18,19,20,21,22,23,24,25 have reported different physiological, genetic, and behavioral effects of developmental lead (Pb2+) exposure. This protocol enables toxicologists to use a more holistic toxicological approach, which is essential to understanding how pollutants are risk factors for both humans and wildlife in an ever increasingly polluted environment.
Access restricted. Please log in or start a trial to view this content.
Protocol
The following protocol is an experimental protocol used to rear species in the Drosophila genus on contaminated medium when oral ingestion of a toxin is appropriate; other forms of exposure are possible using the Drosophila model11,15,16,26. The methods described in this protocol have been previously described by Hirsch et al.19 and Peterson et al.23,24,25.
1. Set Up Stock Populations of Drosophila in the Research Laboratory
- Set up an environmentally-controlled incubator (or small room) to house stock populations of Drosophila by setting the incubators for a constant temperature, light:dark cycle, and humidity, depending upon the preferences of the test species.
NOTE: Preferred environmental conditions will vary depending upon the native ecology of the species chosen for the study. For example, D. melanogaster is native to sub-Saharan Africa27 and is typically maintained at 25 °C, 12:12 light:dark cycle, and approximately 60% humidity16,18,19,20,21,22,23,24,25,28,29,30. On the other hand, D. montana range extends throughout most of Canada and the midwest USA, a much colder region; therefore, D. montana is typically maintained at 19–20 °C and sometimes a 24 h light regime to simulate conditions during the mating season31. For a more detailed description of the geographic ranges of various species of Drosophila, see the Drosophila Speciation Patterns website32. - Obtain a preferred Drosophila species and/or inbred line from either a stock center (see Table of Materials), another research laboratory upon request, or collect wild, genetically variable populations from the field.
NOTE: The following steps explain the methods to collect wild, genetically variable populations of Drosophila to maintain in the research laboratory. These methods have been modified from Markow and O'Grady33 and Werner and Jaenike34 to collect the widest diversity of species at once, rather than target particular species with one bait source.- Freeze half a dozen ripe bananas in the freezer overnight and defrost before setting bait traps.
- Prepare multiple 1–2 L plastic bottles by cutting a u-shaped slit in the front of the bottle to allow flies to be captured in the bait bottle and not escape. Cap the plastic bottles with their bottle caps so the flies do not escape via the lids.
- Add the defrosted banana to the bottom of the bottles so that the bottom of the bottles contains approximately one-inch of banana. Place a slice of ripe tomato in the bottle. Add a yeast slurry (the leftover yeast from the beer making process) to the banana at the bottom of the bottle so that the banana gets to soak in the yeast slurry.
- Add wooden sticks (in an upright vertical position) to the bottle so the flies have a clean substrate to walk on away from the yeast slurry and banana.
Figure 1 illustrates the final product of these methods. - Hang bait bottles in trees overnight and check every 24 h. Mouth aspirate flies out of bottles and individually place females in vials with medium to create iso-female lines.
NOTE: Multi-female lines can be created, however, only if the females of each species can be clearly identified. In addition, flies within the genus Drosophila occupy different ecological niches and will have different dietary requirements depending upon those niches (Werner and Jaenike34); see Werner and Jaenike34 for diet recommendations and food recipes. - Examine the adult F1 offspring under the dissection microscope to identify the species of the collected Drosophila (see Markow and O'Grady33 and Werner and Jaenike34 for assistance in identifying various species).

Figure 1: Pictorial representation of traps and bait used to collect wild populations of Drosophila in the field. (A) Fly traps set at a local field site in Colorado. (B) A closer view of the fly traps set at this field site. Please click here to view a larger version of this figure.
- Maintain the iso-female or the multi-female lines in an environmentally-controlled incubator or room with constant temperature, light:dark cycle, and humidity. To do this, house flies in vials or bottles in preferred medium and allow the gravid females to lay eggs in the medium. Monitor the vials for the presence of larvae and pupae.
NOTE: Flies within the genus Drosophila occupy different ecological niches and will have different dietary requirements and environmental abiotic preferences depending upon those niches33,34. Environmental preferences and dietary recommendations (and further instruction on fly husbandry) can be found in Elgin and Miller28, Shaffer et al.29, Stocker and Gallant30, Markow and O'Grady33, and Werner and Jaenike34. If using wild-caught species, local environmental conditions can be simulated in the incubators until the species can be identified. - Transfer stocks frequently to fresh medium, discarding old vials, to maintain healthy lines and avoid infection from mites.
NOTE: The frequency of transfer will depend on the life cycle of the species. For example, transfer Drosophila melanogaster every 2 weeks to fresh medium.For further information on maintaining lines in the laboratory, see Rand et al.16, Elgin and Miller28, Shaffer et al.29, Stocker and Gallant30, Greenspan35, and Science Education Database36.
2. Rear Drosophila in the Contaminated Medium
NOTE: If testing Drosophila in the laboratory for the first time or with a new contaminant(s), identify the lethal dose (see Castaneda et al.37 and Massie et al.38 for methods) and the LD50 (see Castaneda et al.37 and Akins et al.39 for methods) first. Then, run a dose-response curve to identify biologically-relevant concentrations for the desired phenotypic output; see Hirsch et al.19 and Zhou et al.40 for methods.
- Prepare stock solutions of the contaminated medium at the desired concentration(s), depending upon the chemistry of the contaminant.
NOTE: For example, to prepare stock solutions of PbAc: Prepare stock solutions of lead acetate (PbAc) medium by adding contaminant to distilled water (dH20) until medium made with contaminant water reaches desired concentration. For example, a stock solution of 1,000 µM PbAc, can be prepared by adding PbAc to dH20 until it reaches 1,000 µM PbAc. Further, dilute the stock solution (e.g. the 1,000 µM PbAc solution) to the desired concentration (such as 500 µM PbAc) and maintain these solutions as stock as well. - Prepare medium, following manufacturer's guidelines to serve as the control medium. Prepare additional medium, following manufacturer's guidelines; however, supplement prepared contaminant solution for dH20.
NOTE: For example, if using an instant Drosophila medium, add approximately one teaspoon instant medium to a plastic vial. Add approximately 5–5.5 mL dH20 to the medium. Sprinkle a few grains of live baker's yeast to prepare control medium. To prepare experimental medium, supplement the stock solution (such as 500 µM PbAc) for dH20. - Transfer reproductively viable mature males and females from stock populations into the control and the experimental medium.
NOTE: The time post-eclosion to reproductive maturity is different between the Drosophila species41.- Gently tap the vial of stock flies down with the dominant hand. Ensure that the flies automatically move to the bottom of the vial. With the other hand, remove the cap of the vial while tapping the vial and place a fresh vial of control or contaminated medium on top of the vial with the flies. Hold the vials together and flip them over, gently tapping, so that the flies automatically are transferred to the fresh vial of control or contaminated medium. While still tapping the vial with the flies, cap the vial.
- Repeat with more vials, making sure to standardize the number of flies in each vial.
NOTE: The total number of adults transferred via single transfer or anesthesia will depend on the size of the vials used to avoid overcrowding. - Incubate adults in a standard environmental condition (i.e. an incubator) and allow the adults to mate and lay eggs in the medium for 24–96 h.
- After 24–96 h, discard adults in a morgue (a flask filled with mineral oil and capped with a tight-fitting funnel) leaving behind fertilized eggs (which will later become the experimental subjects) to mature for testing. Place the vials in the incubator to allow the eggs to develop.
- Monitor the vials for wandering-instar larvae by looking for larvae that are emerging from the medium.
3. Collect Experimental Subjects at Various Developmental Stages
NOTE: Experimental subjects can be collected at any developmental stage, placed in the blind coded 15-mL conical tubes, and tested for accumulation. Methods for testing the accumulation of contaminants will depend on the contaminant being studied. For example, accumulation of PbAc can be tested using Inductively-Coupled Plasma Mass Spectrometry (ICP-MS)42. In addition, experimental subjects can be collected at any developmental stage to be tested for a variety of phenotypic effects of contaminants. Figure 2 illustrates the Drosophila life cycle43. Figure 3 illustrates the experimental protocol for exposure and the different developmental stages for collection.
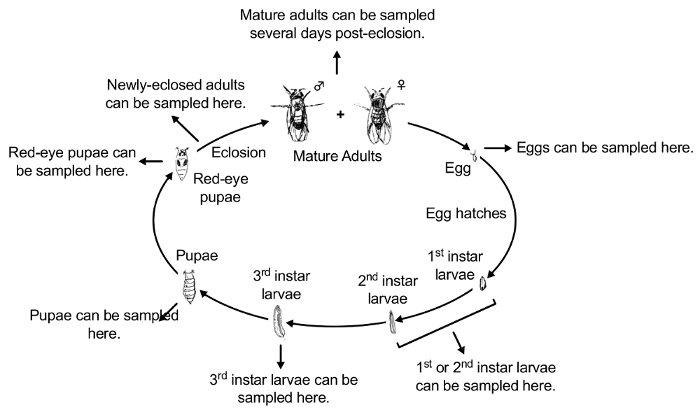
Figure 2: Conceptual overview of the life cycle of D. melanogaster (the most commonly used Drosophila model system). The stages of Drosophila life cycle are: 1) egg, 2) first-instar larva, 3) second-instar larva, 4) third-instar larva, 5) wandering third-instar larva, 6) white-eye pupa, 7) red-eye pupa, 8) newly-eclosed adult, and 9) mature adult. Please click here to view a larger version of this figure.
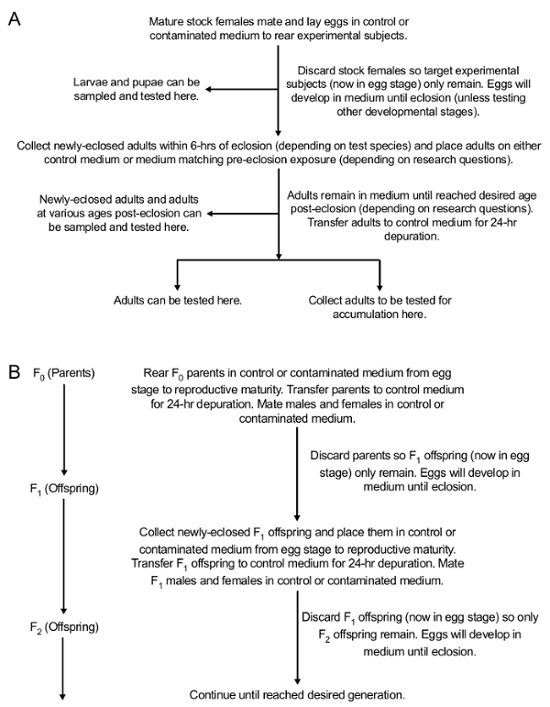
Figure 3: Conceptual overview of the methods for orally exposing Drosophila to contaminated medium in both the parental (F0) and subsequent generations (F1 and onward). (A) Methods for oral exposure during development in the exposed generation. (B) Methods to test the transfer of contaminants to offspring (F1 to the desired generation). This figure has been modified from Peterson et al.24 Please click here to view a larger version of this figure.
- Collect wandering-third instar larvae
- Start monitoring vials when lights turn on in the incubator, as larvae will emerge from the medium and move upwards on the side of the vial within an h after lights turn on in the incubator. Within this h, remove the wandering-third instar larvae from the sides of the vial carefully using a wooden stick or tweezers.
NOTE: The number of larvae available for collection will depend on the number of eggs laid in "2.3.4". - To remove excess medium from the larvae, place the larvae in a small beaker with dH2O. Pour the dH2O out of the beaker and place the larvae on a delicate task wiper. Using a delicate task wiper, gently remove the excess dH2O from the larvae.
- Maintain experimental populations in an environmentally-controlled incubator.
- Start monitoring vials when lights turn on in the incubator, as larvae will emerge from the medium and move upwards on the side of the vial within an h after lights turn on in the incubator. Within this h, remove the wandering-third instar larvae from the sides of the vial carefully using a wooden stick or tweezers.
- Collect newly-eclosed adults
- Monitor vials for eclosion by observing the coloration of the pupae along the sides of the vials.
NOTE: Pupae will darken during development. Developmental time, particularly pre-eclosion, depends on the species tested. - When the first adults begin to eclose, dump and discard these adults into a morgue containing mineral oil.
- When the lights turn on in the incubator the following morning, dump and discard any adults of unknown age (or virginity) that may have eclosed overnight or during the morningbefore lights on.
- Approximately 4 h later, anesthetize any adults that emerged as newly-eclosed adults with a CO2 gun in the vials. Place adults on a CO2 plate under a dissection microscope. Sex adults by looking for sex combs on the forelimbs of males and ovipositors in females.
NOTE: D. melanogaster must be collected within 6 h of eclosion to avoid mating but other species may have longer developmental times (and therefore, do not need to be collected within this time frame). - Separate adults on the CO2 plate using a wooden stick. Gently transfer adults in sex-specific groups using a wooden stick to the medium matching pre-existing history.
- Monitor vials for eclosion by observing the coloration of the pupae along the sides of the vials.
- Collect mature adults post-eclosion
- Allow adults to remain on the medium matching pre-eclosion exposure from egg stage to the desired age post-eclosion in an environmentally-controlled incubator.
- Singly transfer adults to the control medium for 24 h prior to testing to allow adults to groom excess contaminated medium off their bodies.
4. Rear Experimental Subjects to Test the Effects of Multigenerational or Transgenerational Exposure.
- To rear the parental generation (a.k.a the P0 or F0 generations), transfer adults from stock populations to control and the experimental medium following the steps in "2.1" to "2.3" and "3.1" to "3.3".
- When the adults are reproductively mature (see Pitnick et al.41), singly transfer (as stated in 2.3.1) one vial of males to a fresh vial of control or experimental medium. Singly transfer one vial of females to the fresh vial that now contains males. Allow adults to mate and lay eggs in the medium for 24-96 h. Dump and discard adults into a morgue containing mineral oil and re-incubate vials to allow offspring to develop.
- Repeat steps 4.1 through 4.2 depending on the desired number of generations.
Access restricted. Please log in or start a trial to view this content.
Results
By orally exposing Drosophila to a contaminant(s) throughout development, various toxicological questions can be tested by exposing Drosophila at different levels of biological organization. This section presents representative results obtained using this protocol in previously published papers23,24. In particular, this protocol was previously used to evaluate the accumulation, elimination and sequestration of le...
Access restricted. Please log in or start a trial to view this content.
Discussion
Drosophila melanogaster has been established as a powerful model for a range of biological processes due to the extensive conservation of genes and pathways between D. melanogaster and humans13,14. For the same reasons that it's a powerful model for medical science, Drosophila has emerged as a suitable model system to study the impact of anthropogenic pollution on a range of toxicological endpoints. Several laboratories are successf...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This publication was supported by a grant from the Department of Education (PR Award #P031C160025-17, Project title: 84.031C) to the Colorado State University-Pueblo (CSU-Pueblo) Communities to Build Active STEM Engagement (C-BASE). We thank Current Zoology and Elsevier for providing the rights to use the representative results published in previous papers, as well as the editors of JoVE for providing us with the opportunity to publish this protocol. We would also like to thank the C-BASE Program, Dr. Brian Vanden Heuvel (C-BASE and Department of Biology, CSU-Pueblo), CSU-Pueblo Biology department, Thomas Graziano, Dr. Bernard Possidente (Department of Biology, Skidmore College), and Dr. Claire Varian Ramos (Department of Biology, Colorado State University-Pueblo) for their support and assistance.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Carolina Biological Instant Drosophila Medium Formula 4-24 | Carolina Biological | 173204 | |
| Drosophila vials, Narrow (PS), Polystyrene, Superbulk, 1000 vials/unit | Genessee Scientific | 32-116SB | Used to store flies |
| Flugs Closures for vials and bottles, Narrow plastic vials | Genessee Scientific | 49-102 | Used to store flies |
| Cardboard trays, trays only, narrow | Genessee Scientific | 32-124 | Used to organize populations of flies |
| Cardboard trays, dividers only, narrow | Genessee Scientific | 32-126 | Used to organize populations of flies |
| Thermo Scientific Nalgene Square Wide-Mouth HDPE Bottles with Closure | Fischer Scientific | 03-312D | Useful for storage of contaminants |
| Thermo Scientific Nalgene Color-Coded LDPE Wash Bottles | Fischer Scientific | 03-409-17C | Useful for storage of contaminants |
| Eppendorf Repeater M4 Manual Handheld Pipette Dispenser | Fischer Scientific | 14-287-150 | Used to prepare medium |
| Combitips Advanced Pipetter Tips - Standard, Eppendorf Quality Tips | Fischer Scientific | 13-683-708 | Used to prepare medium |
| Flypad, Standard Size (8.1 X 11.6cm) | Genessee Scientific | 59-114 | Used to anesthetize flies |
| Flystuff foot valve | Genessee Scientific | 59-121 | Used to anesthetize flies |
| Tubing, green (1 continguous foot/unit) | Genessee Scientific | 59-124G | Used to anesthetize flies |
| Mineral Oil, Light, White, High Purity Grade, 500 mL HDPE Bottle | VWR | 97064-130 | Used to make a morgue |
| Glass Erlenmeyer Flask Set - 3 Sizes - 50, 150 and 250ml, Karter Scientific 214U2 | Walmart | Not applicable | Used to make a morgue |
| BGSET5 Glass Beaker Set Of 5 | Walmart | ||
| Inbred or wildtype line of Drosophila | Bloomington Drosophila Stock Center at Indiana University | https://bdsc.indiana.edu | |
| Wild popultions of Drosophila | UC San Diego Drosophila Stock Center | https://stockcenter.ucsd.edu/info/welcome.php |
References
- Postel, S. Defusing the Toxics Threat: Controlling Pesticides and Industrial Waste. , Worldwatch Institute. Washington, DC. (1987).
- Vitousek, P. M., Mooney, H. A., Lubchenco, J., Melillo, J. M. Human domination of earth's ecosystems. Science. 277, 494-499 (1997).
- United Nations Environment Program (UNEP). Saving Our Planet: Challenges and Hopes. , UNEP. Nairobi. (1992).
- Hansen, L. J., Johnson, M. L. Conservation and toxicology: Integrating the disciplines. Conservation Biology. 13, 1225-1227 (1999).
- Johnston, E. L., Mayer-Pinto, M., Crowe, T. P. REVIEW: Chemical contaminant effects on marine ecosystem functioning. Journal of Applied Ecology. 52, 140-149 (2015).
- Dell'Omo, G. Behavioral ecotoxicology. , John Wiley & Sons, LTD. West. Sussex, UK. (2002).
- Clotfelter, E. D., Bell, A. M., Levering, K. R. The role of animal behaviour in the study of endocrine-disrupting chemicals. Animal Behaviour. 68, 665-676 (2004).
- Peterson, E. K., Buchwalter, D. B., Kerby, J. L., LeFauve, M. K., Varian-Ramos, C. W., Swaddle, J. P. Integrative behavioral ecotoxicology: bringing together fields to establish new insight to behavioral ecology, toxicology, and conservation. Current Zoology. 63, 185-194 (2017).
- Scott, G. R., Sloman, K. A. The effects of environmental pollutants on complex fish behaviour: Integrating behavioural and physiological indicators of toxicity. Aquatic Toxicology. 68, 369-392 (2004).
- Zala, S. M., Penn, D. J. Abnormal behaviors induced by chemical pollution: A review of the evidence and new challenges. Animal Behaviour. 68, 649-664 (2004).
- Abolaji, A. O., Kamdem, J. P., Farombi, E. O., Rocha, J. B. T. Drosophila melanogaster as a promising model organism in toxicological studies. Archives of Basic & Applied Medicine. 1, 33-38 (2013).
- Jennings, B. H. Drosophila-a versatile model in biology and medicine. Materials Today. 14, 190-195 (2011).
- Pandey, U. B., Nichols, C. D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacology Reviews. 63, 411-436 (2011).
- Rubin, G. M., et al. Comparative genomics of the eukaryotes. Science. 287, 2204-2215 (2000).
- Rand, M. D. Drosophotoxicology: The growing potential for Drosophila in neurotoxicology. Neurotoxicol Teratol. 32, 74(2010).
- Rand, M. D., Montgomery, S. L., Prince, L., Vorojeikina, D. Developmental toxicity assays using the Drosophila model. Current Protocols in Toxicology. 59, 1.12.1-1.12.20 (2015).
- Burke, M. K., Rose, M. R. Experimental evolution with Drosophila. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 296, R1847-R1854 (2009).
- He, T., Hirsch, H. V. B., Ruden, D. M., Lnenicka, G. A. Chronic lead exposure alters presynaptic calcium regulation and synaptic facilitation in Drosophila larvae. NeuroToxicology. 30, 777-784 (2009).
- Hirsch, H. V., et al. Behavioral effects of chronic exposure to low levels of lead in Drosophila melanogaster. NeuroToxicology. 24, 435-442 (2003).
- Hirsch, H. V. B., et al. Variations at a quantitative trait locus (QTL) affect development of behavior in lead-exposed Drosophila melanogaster. NeuroToxicology. 30, 305-311 (2009).
- Morley, E. J., Hirsch, H. V. B., Hollocher, K., Lnenicka, G. A. Effects of chronic lead exposure on the neuromuscular junction in Drosophila larvae. NeuroToxicology. 24, 35-41 (2003).
- Ruden, D. M., et al. Genetical toxicologenomics in Drosophila identifies master- modulatory loci that are regulated by developmental exposure to lead. NeuroToxicology. 30, 898-914 (2009).
- Peterson, E. K., et al. Accumulation, elimination, sequestration, and genetic variation of lead (Pb2+) loads within and between generations of Drosophila melanogaster. Chemosphere. 181, 368-375 (2017).
- Peterson, E. K., et al. Asymmetrical positive assortative mating induced by developmental lead (Pb2+) exposure in a model system, Drosophila melanogaster. Current Zoology. 63, 195-203 (2017).
- Peterson, E. K. Consequences of developmental lead (Pb2+) exposure on reproductive strategies in Drosophila. , University at Albany-State University of New York. Dissertation (2016).
- Chifiriuc, M. C., Ratiu, A. C., Popa, M., Ecovolu, A. A. Drosophotoxicology: An emerging research area for assessing nanoparticles interaction with living organisms. International Journal of Molecular Sciences. 17, 36(2016).
- Lachaise, D., Cariou, M. L., David, J. R., Lemeunier, F., Tsacas, L., Ashburner, M. Historical biogeography of the Drosophila melanogaster species subgroup. Evolutionary Biology. 22, 159-225 (1988).
- Elgin, C. R., Miller, D. W. Mass rearing of flies and mass production and harvesting of embryos. The Genetics and Biology of Drosophila. Ashburner, M., Wright, T. R. F. 2a, 112-121 (1978).
- Shaffer, C. D., Wuller, J. M., Elgin, C. R. Chapter 5: Raising large quantities of Drosophila for biochemical experiments. Methods in Cell Biology. 44, 99-108 (1994).
- Stocker, H., Gallant, P. Getting started: an overview on raising and handling Drosophila. Methods in Molecular Biology. 420, 27-44 (2008).
- Jennings, J. H., Etges, W. J., Schmitt, T., Hoikkala, A. Cuticular hydrocarbons of Drosophila montana: geographic variation, sexual dimorphism and potential roles as pheromones. Journal of Insect Physiology. 61, 16-24 (2014).
- Drosophila Speciation Patterns. , http://www.drosophila-speciation-patterns.com/rangemaps.html. (2018).
- Markow, T. A., O'Grady, P. M. Drosophila: A Guide to Species Identification and Use. , Academic Press. London. (2005).
- Werner, T., Jaenike, J. Drosopholids of the midwest and northeast. , River Campus Libraries, University of Rochester. Rochester NY. (2017).
- Greenspan, R. J. The basics of doing a cross. Fly Pushing: The theory and practice of Drosophila genetics. , 2nd, Cold Spring Harbor Laboratory Press, Cold Spring Harbor. New York. 3-24 (1997).
- JoVE Science Education Database. . Biology I: yeast, Drosophila and C. elegans. Drosophila Maintenance. , JoVE. Cambridge, MA. (2018).
- Castañeda, P. L., Muñoz, G. L. E., Durán, D. A., Heres, P. M. E., Dueñas, G. I. E. LD50 in Drosophila melanogaster. fed on lead nitrate and lead acetate. Drosophila Information Service. 84, 44-48 (2001).
- Massie, H. R., Aiello, V. R., Whitney, S. J. P. Lead accumulation during aging of Drosophila and effect of dietary lead on life span. Age. 15, 47-49 (1992).
- Akins, J. M., Schroeder, J. A., Brower, D. L., Aposhian, H. V. Evaluation of Drosophila melanogaster as an alternative animal for studying the neurotoxicity of heavy metals. BioMetals. 5, 111-120 (1992).
- Zhou, S., et al. The genetic basis for variation in sensitivity to lead toxicity in Drosophila melanogaster. Environmental Health Perspectives. 124, 1062-1070 (2016).
- Pitnick, S., Markow, T. A., Spicer, G. S. Delayed male maturity is a cost of producing large sperm in Drosophila. Proceedings of National Academy of Sciences USA. 92, 10614-10618 (1995).
- Beauchemin, D. Inductively Coupled Plasma Mass Spectrometry. Analytical Chemistry. 82, 4786-4810 (2010).
- Tyler, M. S. Development of the fruit fly Drosophila melanogaster. Developmental Biology, a Guide for Experimental Study. Tyler, M. S. , 2nd, Sinauer Associates Inc. Sunderland, MA, USA. 8-27 (2000).
- Ortiz, J. G., Opoka, R., Kane, D., Cartwright, I. L. Investigating arsenic susceptibility from a genetic perspective in Drosophila reveals a key role for glutathione synthetase. Toxicological Sciences. 107, 416-426 (2009).
- Bonilla, E., Contreras, R., Medina-Leendertz, S., Mora, M., Villalobos, V., Bravo, Y. Minocycline increases the life span and motor activity and decreases lipid peroxidation in manganese treated Drosophila melanogaster. Toxicology. 294, 50-53 (2012).
- Guarnieri, D. J., Heberlein, U. Drosophila melanogaster, a genetic model system for alcohol research. International Review of Neurobiology. 54, 199-228 (2003).
- Posgai, R., Cipolla-McCulloch, C. B., Murphy, K. R., Hussain, S. M., Rowe, J. J., Nielsen, M. G. Differential toxicity of silver and titanium dioxide nanoparticles on Drosophila melanogaster development, reproductive effort, and viability: size, coatings and antioxidants matter. Chemosphere. 85, 34-42 (2011).
- Gupta, S. C., et al. Adverse effect of organophosphate compounds, dichlorvos and chlorpyrifos in the reproductive tissues of transgenic Drosophila melanogaster: 70kDa heat shock protein as a marker of cellular damage. Toxicology. 238, 1-14 (2007).
- Wasserkort, R., Koller, T. Screening toxic effects of volatile organic compounds using Drosophila melanogaster. Journal of Applied Toxicology. 17, 119-125 (1997).
- Markow, T. A., O'Grady, P. O. Reproductive ecology of Drosophila. Functional Ecology. 22, 747-759 (2008).
- Dev, K., Chahal, J., Parkash, R. Seasonal variations in the mating-related traits of Drosophila melanogaster. Journal of Ethology. 31, 165-174 (2013).
- Salminen, T. S., Vesala, L., Laiho, A., Merisalo, M., Hoikkala, A., Kankare, M. Seasonal gene expression kinetics between diapause phases in Drosophila virilus group species and overwintering differences between diapausing and non-diapausing females. Nature Scientific Reports. 5, 11197(2015).
- Miller, R. S., Thomas, J. L. The effects of larval crowding and body size on the longevity of adult Drosophila melanogaster. Ecology. 39, 118-125 (1958).
- Peterson, E. K., Ghiradella, H., Possidente, B., Hirsch, H. Transgenerational epigenetic effects of lead exposure on behavior in Drosophila melanogaster. Abstracts of the IBANGS Genes, Brain and Behavior Meeting, May 16-19, 2012, Boulder, CO, 11, Genes, Brain & Behavior 492-493 (2012).
- Soares, J. J., et al. Continuous liquid feeding: New method to study pesticides toxicity in Drosophila melanogaster. Analytical Biochemistry. 537, 60-62 (2017).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved