A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Fabricating a Kidney Cortex Extracellular Matrix-Derived Hydrogel
In This Article
Summary
Here we present a protocol to fabricate a kidney cortex extracellular matrix-derived hydrogel to retain the native kidney extracellular matrix (ECM) structural and biochemical composition. The fabrication process and its applications are described. Finally, a perspective on using this hydrogel to support kidney-specific cellular and tissue regeneration and bioengineering is discussed.
Abstract
Extracellular matrix (ECM) provides important biophysical and biochemical cues to maintain tissue homeostasis. Current synthetic hydrogels offer robust mechanical support for in vitro cell culture but lack the necessary protein and ligand composition to elicit physiological behavior from cells. This manuscript describes a fabrication method for a kidney cortex ECM-derived hydrogel with proper mechanical robustness and supportive biochemical composition. The hydrogel is fabricated by mechanically homogenizing and solubilizing decellularized human kidney cortex ECM. The matrix preserves native kidney cortex ECM protein ratios while also enabling gelation to physiological mechanical stiffnesses. The hydrogel serves as a substrate upon which kidney cortex-derived cells can be maintained under physiological conditions. Furthermore, the hydrogel composition can be manipulated to model a diseased environment which enables the future study of kidney diseases.
Introduction
Extracellular matrix (ECM) provides important biophysical and biochemical cues to maintain tissue homeostasis. The complex molecular composition regulates both structural and functional properties of tissue. Structural proteins provide cells with spatial awareness and allow for adhesion and migration1. Bound ligands interact with cell surface receptors to control cell behavior2. Kidney ECM contains a plethora of molecules whose composition and structure varies depending on anatomical location, developmental stage, and disease state3,4. Recapitulating the complexity of ECM is a key aspect in studying kidney-derived cells in vitro.
Previous attempts at replicating ECM microenvironments have focused on decellularizing whole tissue to create scaffolds capable of recellularization. Decellularization has been performed with chemical detergents such as sodium dodecyl sulfate (SDS) or non-ionic detergents, and it utilizes either whole organ perfusion or immersion and agitation methods5,6,7,8,9,10,11,12,13. The scaffolds presented here preserve the structural and biochemical cues found in native tissue ECM; furthermore, recellularization with donor-specific cells has clinical relevance in reconstructive surgery14,15,16,17,18,19. However, these scaffolds lack structural flexibility and are therefore incompatible with many current devices used for in vitro studies. To overcome this limitation, many groups have further processed decellularized ECM into hydrogels20,21,22,23,24. These hydrogels are compatible with injection molding and bioink and circumvent micrometer scale spatial constraints that decellularized scaffolds place on cells. Furthermore, molecular composition and ratios found in native ECM are preserved3,25. Here we demonstrate a method to fabricate a hydrogel derived from kidney cortex ECM (kECM).
The purpose of this protocol is to produce a hydrogel that replicates the microenvironment of the kidney cortical region. Kidney cortex tissue is decellularized in a 1% SDS solution under constant agitation to remove cellular matter. SDS is commonly used to decellularize tissue because of its ability to quickly remove immunological cellular material6,7,9,26. The kECM is then subject to mechanical homogenization and lyophilization5,6,9,11,26. Solubilization in a strong acid with pepsin results in a final hydrogel stock solution20,27. Native kECM proteins that are important for structural support and signal transduction are preserved3,25. The hydrogel can also be gelled to within one order of magnitude of native human kidney cortex28,29,30. This matrix provides a physiological environment that has been used to maintain the quiescence of kidney-specific cells compared to hydrogels from other matrix proteins. Furthermore, matrix composition can be manipulated, for example, through the addition of collagen-I, to model disease environments for the study of renal fibrosis and other kidney diseases31,32.
Protocol
Human kidneys were isolated by LifeCenter Northwest following ethical guidelines set by the Association of Organ Procurement Organizations. This protocol follows animal care and cell culture guidelines outlined by the University of Washington.
1. Preparation of Human Kidney Tissue
- Preparation of decellularization solution
- Sterilize a 5000 mL beaker and a 70 x 10 mm stir bar.
- Mix 1:1000 (weight:volume) sodium dodecyl sulfate (SDS) in autoclaved deionized water in the beaker. Leave the solution on a stir plate at approximately 200 rpm for 24 h or until the SDS is completely dissolved.
Note: Typically, 2500 mL of 1% SDS solution is sufficient to decellularize a single human kidney. - Transfer the solution to a 500 mL sterile vacuum filter and filter it into sterilized sealable containers.
- Processing of kidney tissue
- Wash and autoclave a pair of forceps, two hemostat clamps, a pair of general service grade scissors, two scalpel blade handles, a 1000 mL beaker covered with aluminum foil, and a 36 x 9 mm stir bar.
- Line a tissue culture hood with underpad. Place the beaker, a sterile tissue culture dish (150 x 25 mm), and the whole kidney organ into the hood. Fill the beaker with 500 mL of 1% SDS solution.
Note: Human kidneys were received on ice from LifeCenter NorthWest. - Place the kidney in the sterile tissue culture dish (Figure 1A). Remove all perirenal fat by lightly shaving around the renal capsule with a scalpel (Figure 1B).
- Make a shallow 8-10 cm incision with the scalpel, just deep enough to break open the renal capsule without damaging the underlying cortex tissue, across the superior end of the kidney. Remove the renal capsule by peeling it away from the cortex tissue with two hemostat clamps (Figure 1C).
- Bisect the kidney along the coronal plane by using the scalpel along the lateral side of the kidney (Figure 1D). Isolate cortex tissue from both halves by carving out the medullar region with the scalpel (Figure 1E) and dice the cortex tissue into 0.5 cm3 pieces (Figure 1F). Remove any large visible vessels.
- Isolation of extracellular matrix
- In a tissue culture hood, fill a 1000 mL beaker with 500 mL of 1% SDS solution. Place the diced cortex tissue and stir bar into the beaker containing SDS solution. Cover the beaker with autoclaved aluminum foil and place it on a stir plate at approximately 400 rpm outside of the tissue culture hood.
- After the cortex tissue has been on the stir plate for 24 h, bring the beaker into a tissue culture hood and add a 40 µm sterile cell strainer made with nylon mesh. Fill a separate 1000 mL beaker with 200 mL of bleach and place it in the tissue culture hood.
- Pipette out the SDS solution through the cell strainer into the beaker containing bleach. Pipette out all SDS solution until only decellularized tissue and the cell strainer remain in the beaker.
Note: The cell strainer should prevent any tissue from being removed during solution aspiration. - Leave the cell strainer in the beaker and fill with 500 mL of fresh SDS solution. Cover the beaker with the same aluminum foil and place onto a stir plate at the same speed as before.
- Repeat steps 1.3.1-1.3.3 every 24 hours with fresh SDS solution for a total of five days.
- Rinse decellularized tissue with autoclaved DI water every 24 h for 3 days total, following the technique outlined in steps 1.3.1-1.3.3.
- Rinse decellularized tissue with cell culture grade water every 24 h for 2 days total, following the technique outlined in steps 1.3.1-1.3.3.
- Repeat steps 1.3.1-1.3.2. Transfer the decellularized tissue (referred to as kECM from this point on) into a 30 mL self-standing conical tube and fill it with cell culture grade water until all the tissue is submerged.
2. Fabrication of Hydrogel Stock Solution
- Mechanical processing of decellularized tissue
- In a tissue culture hood, mechanically homogenize the kECM within the conical tube with a tissue homogenizer for 2 min.
Note: Homogenized kECM should resemble an opaque solution with no visible pieces of ECM. - Submerge the conical tube containing the kECM in liquid nitrogen until boiling surrounding the tube no longer persists. Store the kECM at -4 ˚C overnight.
- In a tissue culture hood, mechanically homogenize the kECM within the conical tube with a tissue homogenizer for 2 min.
- Lyophilization of frozen decellularized tissue
- Slightly loosen the conical tube cap to allow for gas exchange and place the tube into a lyophilization machine. Lyophilize the kECM for three days or until it resembles a fine white powder. Store at -4 ˚C.
- Chemical digestion and solubilization of gel
- Autoclave a 20 mL scintillation vial and cap, a 15.9 x 7.9 mm stir bar, and one pair of fine-tip forceps.
- Weigh the lyophilized kECM and calculate the volume of HCl and mass of pepsin needed to solubilize the kECM to a 3% (30 mg/mL) solution using the following equations, where mpepsin is the mass of pepsin, mtissue is the mass of lyophilized tissue, and VHCl is the volume of 0.01 N HCl:
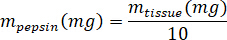
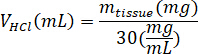
- In a tissue culture hood, add porcine gastric pepsin, 0.01 N HCl, and the stir bar to the scintillation vial, and leave it on a stir plate at approximately 500 rpm until all the pepsin has dissolved. Transfer the lyophilized kECM to the scintillation vial and leave the solution on a stir plate at approximately 500 rpm for three days.
3. Hydrogel Gelation
- Kidney ECM hydrogel preparation
- Gel the hydrogel by mixing the kECM hydrogel stock solution with 1 N NaOH, 10x Media Supplement (M199), and cell culture media. Keep all the solutions on ice.
Note: Final gel concentrations of 7.5 mg/mL were used for cell culture. 1 mL of kECM gel was sufficient for cell culture experiments presented. - Determine the volume of workable kECM gel produced and volume of stock kECM hydrogel needed by using the following equation, where Vfinal is the volume of gel created, Vstock kECM is the volume of stock kECM hydrogel needed, Cstock kECM is the concentration of the stock kECM hydrogel, and Cfinal is the concentration of the final gel:
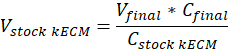
- Determine the volume of neutralizing reagents needed by using the following equations, where VNaOH is the volume of 1 N NaOH, V10X is the volume of M199 10X media supplement, and V1X is the volume of cell culture media:
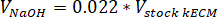
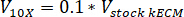
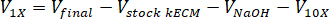
- In a tissue culture hood, pipette the neutralizing reagents (NaOH, M199, and cell culture media) into a sterile 30 mL self-standing conical tube. Mix the neutralizing reagent solution with a microspatula.
- Use a sterile 1 mL syringe to transfer the appropriate volume of stock kECM hydrogel to the neutralizing reagent solution. Use a microspatula to gently mix the solution until a homogeneous in color hydrogel solution is obtained.
Note: Avoid introducing air bubbles by stirring slowly and gently. - To incorporate cells into the kECM hydrogel, subtract 10 µL of cell culture media (V1X) from the neutralizing solution volume calculations in step 3.1.1.3.
- Suspend cells into 10 µL of cell culture media. Determine the number of cells to be suspended by using the following equation, where #cells implies the number of cells to suspend and Vfinal is the volume of gel created:
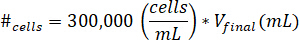
Note: 300,000 cells/mL is the concentration of cells used in the kECM gel. - Pipette the 10 µL of cell suspended solution into the final kECM gel after the kECM stock solution has been mixed with neutralizing reagent solution. Stir the solution with a microspatula until the cells are evenly distributed.
- Suspend cells into 10 µL of cell culture media. Determine the number of cells to be suspended by using the following equation, where #cells implies the number of cells to suspend and Vfinal is the volume of gel created:
- Gel the hydrogel by mixing the kECM hydrogel stock solution with 1 N NaOH, 10x Media Supplement (M199), and cell culture media. Keep all the solutions on ice.
- Use a 1 mL syringe to fill a desired cell culture device with the kECM hydrogel.
- Allow the gel to set at 37 ˚C for 1 h before transferring or plating cells.
Results
The kECM hydrogel provides a matrix for kidney cell culture with similar chemical composition as the native kidney microenvironment. To fabricate the hydrogel, kidney cortex tissue is mechanically isolated from a whole kidney organ and diced (Figure 1). Decellularization with a chemical detergent (Figure 2A.1-A.3) followed by rinses with water to remove detergent particles (Figure 2A...
Discussion
Matrices provide important mechanical and chemical cues that govern cell behavior. Synthetic hydrogels are able to support complex 3-dimensional patterning but fail to provide the diverse extracellular cues found in physiological matrix microenvironments. Hydrogels derived from native ECM are ideal materials for both in vivo and in vitro studies. Previous studies have used decellularized ECM hydrogels to coat synthetic biomaterials to prevent host immunological responses33,<...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the Lynn and Mike Garvey Imaging Laboratory at the Institute for Stem Cell and Regenerative Medicine and LifeCenter NorthWest. They would also like to acknowledge the financial support of National Institutes of Health grants, UH2/UH3 TR000504 (to J.H.) and DP2DK102258 (to Y.Z.), NIH T32 training grant DK0007467 (to R.J.N.), and an unrestricted gift from the Northwest Kidney Centers to the Kidney Research Institute.
Materials
| Name | Company | Catalog Number | Comments |
| Preparation of Kidney Tissue | |||
| 5000 mL Beaker | Sigma-Aldrich | Z740589 | |
| Sodium Dodecyl Sulfate (SDS) | Sigma-Aldrich | 436143 | |
| Sterile H2O | Autoclaved DI H2O | ||
| Stir Bar (70 x 10 mm) | Fisher Science | 14-512-128 | |
| 500 mL Vacuum Filter | VWR | 97066-202 | |
| Stir Plate | Sigma-Aldrich | CLS6795420D | |
| 1000 mL Beaker | Sigma-Aldrich | CLS10031L | |
| Forceps | Sigma-Aldrich | F4642 | Any similar forceps may be used |
| Scissor-Handle Hemostat Clamp | Sigma-Aldrich | Z168866 | |
| Dissecting Scissors | Sigma-Aldrich | Z265977 | |
| Scalpel Handle, No. 4 | VWR | 25859-000 | Any similar scalpel handle may be used |
| Scalpel Blade, No. 20 | VWR | 25860-020 | Any similar scalpel blade may be used |
| Stir Bar (38.1 x 9.5 mm) | Fisher Science | 14-513-52 | |
| Absorbent Underpad | VWR | 82020-845 | |
| Petri Dish (150 x 25 mm) | Corning | 430597 | |
| Autoclavable Biohazard Bag | VWR | 14220-026 | |
| Sterile Cell Strainer (40 um) | Fisher Science | 22-363-547 | |
| Cell Culture Grade Water | HyClone | SH30529.03 | |
| 30 mL Freestanding Tube | VWR | 89012-778 | |
| Fabrication of ECM Gel | |||
| Tissue Homogenizer Machine | Polytron | PCU-20110 | |
| Freeze Dryer | Labconco | 7670520 | |
| 20 mL Glass Scintillation Vials and Cap | Sigma-Aldrich | V7130 | |
| Stir Bar (15.9 x 8 mm) | Fisher Science | 14-513-62 | |
| Pepsin from Porcine Gastric Mucosa | Sigma-Aldrich | P7012 | |
| 0.01 N HCl | Sigma-Aldrich | 320331 | Dilute to 0.01 N HCl with cell culuture water |
| Kidney ECM Gelation | |||
| 1 N NaOH (Sterile) | Sigma-Aldrich | 415413 | Dilute to 1 N in cell culture grade water |
| Medium 199 | Sigma-Aldrich | M4530 | |
| 15 mL Conical Tube | ThermoFisher | 339651 | |
| Cell Culture Media | ThermoFisher | 11330.032 | Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12) |
| Fetal Bovine Serum (FBS) | Gibco | 10082147 | |
| Antibiotic-Antimycotic 100X | Life Technologies | 15240-062 | |
| Insulin, Transferrin, Selenium, Sodium Pyruvate Solution (ITS-A) 100X | Life Technologies | 51300-044 | |
| 1 mL Syringe | Sigma-Aldrich | Z192325 | |
| Microspatula | Sigma-Aldrich | Z193208 |
References
- Lelongt, B., Ronco, P. Role of extracellular matrix in kidney development and repair. Pediatric Nephrology. 18 (8), 731-742 (2003).
- Yue, B. Biology of the Extracellular Matrix: An Overview. Journal of Glaucoma. 23, S20-S23 (2014).
- Miner, J. H. Renal basement membrane components. Kidney International. 56 (6), 2016-2024 (1999).
- Petrosyan, A., et al. Decellularized Renal Matrix and Regenerative Medicine of the Kidney: A Different Point of View. Tissue Engineering Part B. 22 (3), 183-192 (2016).
- Caralt, M., et al. Optimization and Critical Evaluation of Decellularization Strategies to Develop Renal Extracellular Matrix Scaffolds as Biological Templates for Organ Engineering and Transplantation. American Journal of Transplantation. 15 (1), 64-75 (2015).
- Nakayama, K. H., Batchelder, C. A., Lee, C. I., Tarantal, A. F. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Engineering Part A. 16 (7), 2207-2216 (2010).
- Nakayama, K. H., Lee, C. C. I., Batchelder, C. A., Tarantal, A. F. Tissue Specificity of Decellularized Rhesus Monkey Kidney and Lung Scaffolds. Public Library of Science ONE. 8 (5), (2013).
- Orlando, G., et al. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Annals of Surgery. 256 (2), 363-370 (2012).
- Sullivan, D. C., et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 33 (31), 7756-7764 (2012).
- Choi, S. H., et al. Development of a porcine renal extracellular matrix scaffold as a platform for kidney regeneration. Journal of Biomedical Materials Research Part A. 103 (4), 1391-1403 (2015).
- Ross, E. A., et al. Mouse stem cells seeded into decellularized rat kidney scaffolds endothelialize and remodel basement membranes. Organogenesis. 8 (2), 49-55 (2012).
- Nagao, R. J., et al. Decellularized Human Kidney Cortex Hydrogels Enhance Kidney Microvascular Endothelial Cell Maturation and Quiescence. Tissue Engineering Part A. 22 (19-20), 1140-1150 (2016).
- Gupta, S. K., Mishra, N. C., Dhasmana, A. Decellularization Methods for Scaffold Fabrication. Methods in Molecular Biology. , 1-10 (2017).
- Hudson, T., et al. Optimized Acellular Nerve Graft is Immunologically Tolerated and Supports Regeneration. Tissue Engineering. 10 (11), 1641-1651 (2004).
- Atala, A., Bauer, S. B., Soker, S., Yoo, J. J., Retik, A. B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 367 (9518), 1241-1246 (2006).
- Ott, H. C., et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nature Medicine. 14 (2), 213-221 (2008).
- Uygun, B., et al. Organ reengineering through development of a transplantable recellularied liver graft using decellularized liver matrix. Nature Medicine. 16 (7), 814-820 (2010).
- Nagao, R. J., et al. Preservation of Capillary-beds in Rat Lung Tissue Using Optimized Chemical Decellularization. Journal of Materials Chemistry B. 1 (37), 4801-4808 (2013).
- Song, J. J., et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nature Medicine. 19 (5), 646-651 (2013).
- Freytes, D. O., Martin, J., Velankar, S. S., Lee, A. S., Badylak, S. F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 29 (11), 1630-1637 (2008).
- Wolf, M. T., et al. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. 33 (29), 7028-7038 (2012).
- Fisher, M. B., et al. Potential of healing a transected anterior cruciate ligament with genetically modified extracellular matrix bioscaffolds in a goat model. Knee Surgery, Sports Traumatology, Arthroscopy. 20 (7), 1357-1365 (2012).
- Ghuman, H., et al. ECM hydrogel for the treatment of stroke: Characterization of the host cell infiltrate. Biomaterials. 91, 166-181 (2016).
- Rijal, G. The decellularized extracellular matrix in regenerative medicine. Regenerative Medicine. 12 (5), 475-477 (2017).
- Lennon, R., et al. Global Analysis Reveals the Complexity of the Human Glomerular Extracellular Matrix. Journal of the American Society of Nephrology. 25 (5), 939-951 (2014).
- Bonandrini, B., et al. Recellularization of Well-Preserved Acellular Kidney Scaffold Using Embryonic Stem Cells. Tissue Engineering Part A. 20 (9-10), 1486-1498 (2014).
- O'Neill, J. D., Freytes, D. O., Anandappa, A. J., Oliver, J. A., Vunjak-Novakovic, G. V. The regulation of growth and metabolism of kidney stem cells with regional specificity using extracellular matrix derived from kidney. Biomaterials. 34 (38), 9830-9841 (2013).
- Streitberger, K. -. J., et al. High-resolution mechanical imaging of the kidney. Journal of Biomechanics. 47 (3), 639-644 (2014).
- Bensamoun, S. F., et al. Stiffness imaging of the kidney and adjacent abdominal tissues measured simultaneously using magnetic resonance elastography. Clinical Imaging. 35 (4), 284-287 (2011).
- Moon, S. K., et al. Quantification of Kidney Fibrosis Using Ultrasonic Shear Wave Elastography. Journal of Ultrasound in Medicine. 34, 869-877 (2015).
- Genovese, F., Manresa, A. A., Leeming, D. J., Karsdal, M. A., Boor, P. The extracellular matrix in the kidney: a source of novel non-invasive biomarkers of kidney fibrosis?. Fibrogenesis & Tissue Repair. 7 (1), (2014).
- Hewitson, T. D. Fibrosis in the kidney: is a problem shared a problem halved?. Fibrogenes & Tissue Repair. 5 (1), S14 (2012).
- Wolf, M. T., et al. Polypropylene surgical mesh coated with extracellular matrix mitigates the host foreign body response. Journal of Biomedical Materials Research Part A. 102 (1), 234-246 (2014).
- Faulk, D. M., et al. ECM hydrogel coating mitigates the chronic inflammatory response to polypropylene mesh. Biomaterials. 35 (30), 8585-8595 (2014).
- Jeffords, M. E., Wu, J., Shah, M., Hong, Y., Zhang, G. Tailoring Material Properties of Cardiac Matrix Hydrogels To Induce Endothelial Differentiation of Human Mesenchymal Stem Cells. ACS Applied Materials & Interfaces. 7 (20), 11053-11061 (2015).
- Kim, M. -. S., et al. Differential Expression of Extracellular Matrix and Adhesion Molecules in Fetal-Origin Amniotic Epithelial Cells of Preeclamptic Pregnancy. Public Library of Science ONE. 11 (5), e0156038 (2016).
- Paduano, F., Marrelli, M., White, L. J., Shakesheff, K. M., Tatullo, M. Odontogenic Differentiation of Human Dental Pulp Stem Cells on Hydrogel Scaffolds Derived from Decellularized Bone Extracellular Matrix and Collagen Type I. Public Library of Science ONE. 11 (2), e0148225 (2016).
- Viswanath, A., et al. Extracellular matrix-derived hydrogels for dental stem cell delivery. Journal of Biomedical Materials Research Part A. 105 (1), 319-328 (2017).
- Uriel, S., et al. Extraction and Assembly of Tissue-Derived Gels for Cell Culture and Tissue Engineering. Tissue Engineering Part C Methods. 15 (3), 309-321 (2009).
- Saldin, L. T., Cramer, M. C., Velankar, S. S., White, L. J., Badylak, S. F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomaterialia. 49, 1-15 (2017).
- Faust, A., et al. Urinary bladder extracellular matrix hydrogels and matrix-bound vesicles differentially regulate central nervous system neuron viability and axon growth and branching. Journal of Biomaterials Applications. 31 (9), 1277-1295 (2017).
- Pouliot, R. A., et al. Development and characterization of a naturally derived lung extracellular matrix hydrogel. Journal of Biomedical Materials Research Part A. 104 (8), 1922-1935 (2016).
- Pati, F., et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nature Communications. 5, 3935 (2014).
- Pati, F., et al. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials. 62, 164-175 (2015).
- Wang, R. M., Christman, K. L. Decellularized myocardial matrix hydrogels: In basic research and preclinical studies. Advanced Drug Delivery Reviews. 96, 77-82 (2016).
- Jang, J., et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 112, 264-274 (2017).
- Frantz, C., Stewart, K. M., Weaver, V. M. The extracellular matrix at a glance. Journal of Cell Science. 123 (Pt 24), 4195-4200 (2010).
- Mouw, J. K., Ou, G., Weaver, V. M. Extracellular matrix assembly: a multiscale deconstruction. Nature Reviews Molecular Cell Biology. 15 (12), 771-785 (2014).
- Bonnans, C., Chou, J., Werb, Z. Remodelling the extracellular matrix in development and disease. Nature Reviews Molecular Cell Biology. 15 (12), 786-801 (2014).
- Hinderer, S., Layland, S. L., Schenke-Layland, K. ECM and ECM-like materials - Biomaterials for applications in regenerative medicine and cancer therapy. Advanced Drug Delivery Reviews. 97, 260-269 (2016).
- Uriel, S., et al. The role of adipose protein derived hydrogels in adipogenesis. Biomaterials. 29 (27), 3712-3719 (2008).
- Singelyn, J. M., et al. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 30 (29), 5409-5416 (2009).
- Medberry, C. J., et al. Hydrogels derived from central nervous system extracellular matrix. Biomaterials. 34 (4), 1033-1040 (2013).
- Loneker, A. E., Faulk, D. M., Hussey, G. S., D'Amore, A., Badylak, S. F. Solubilized liver extracellular matrix maintains primary rat hepatocyte phenotype in-vitro. Journal of Biomedical Materials Research Part A. 104 (4), 957-965 (2016).
- Hill, R. C., Calle, E. A., Dzieciatkowska, M., Niklason, L. E., Hansen, K. C. Quantification of extracellular matrix proteins from a rat lung scaffold to provide a molecular readout for tissue engineering. Molecular & Cellular Proteomics. 14 (4), 961-973 (2015).
- Li, Q., et al. Proteomic analysis of naturally-sourced biological scaffolds. Biomaterials. 75, 37-46 (2016).
- Tanaka, T., Yada, R. Y. N-terminal portion acts as an initiator of the inactivation of pepsin at neutral pH. Protein Engineering. 14 (9), 669-674 (2001).
- Ligresti, G., et al. A Novel Three-Dimensional Human Peritubular Microvascular System. Journal of the American Society of Nephrology. 27 (8), 2370-2381 (2016).
- Mozes, M. M., Böttinger, E. P., Jacot, T. A., Kopp, J. B. Renal expression of fibrotic matrix proteins and of transforming growth factor-beta (TGF-beta) isoforms in TGF-beta transgenic mice. Journal of the American Society of Nephrology. 10 (2), 271-280 (1999).
- Romanowicz, L., Galewska, Z. Extracellular matrix remodeling of the umbilical cord in pre-eclampsia as a risk factor for fetal hypertension. Journal of Pregnancy. 2011, 542695 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved