A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Multiplexing Focused Ultrasound Stimulation with Fluorescence Microscopy
In This Article
Summary
Low-Intensity Pulsed Ultrasound Stimulation (LIPUS) is a modality for non-invasive mechanical stimulation of endogenous or engineered cells with high spatial and temporal resolution. This article describes how to implement LIPUS to an epi-fluorescence microscope and how to minimize acoustic impedance mismatch along the ultrasound path to prevent unwanted mechanical artefacts.
Abstract
By focusing low-intensity ultrasound pulses that penetrate soft tissues, LIPUS represents a promising biomedical technology to remotely and safely manipulate neural firing, hormonal secretion and genetically-reprogrammed cells. However, the translation of this technology for medical applications is currently hampered by a lack of biophysical mechanisms by which targeted tissues sense and respond to LIPUS. A suitable approach to identify these mechanisms would be to use optical biosensors in combination with LIPUS to determine underlying signaling pathways. However, implementing LIPUS to a fluorescence microscope may introduce undesired mechanical artefacts due to the presence of physical interfaces that reflect, absorb and refract acoustic waves. This article presents a step-by-step procedure to incorporate LIPUS to commercially-available upright epi-fluorescence microscopes while minimizing the influence of physical interfaces along the acoustic path. A simple procedure is described to operate a single-element ultrasound transducer and to bring the focal zone of the transducer into the objective focal point. The use of LIPUS is illustrated with an example of LIPUS-induced calcium transients in cultured human glioblastoma cells measured using calcium imaging.
Introduction
Many diseases require some form of invasive medical intervention. These procedures are often expensive, risky, require recovery periods and thus add a burden to health care systems. Non-invasive therapeutic modalities have the potential to provide safer and cheaper alternatives to conventional surgical procedures. However, current non-invasive approaches such as pharmacotherapy or transcranial magnetic stimulation are often limited by trade-offs between tissue penetration, spatiotemporal resolution and unwanted off-target effects. In this context, a focused ultrasound constitutes a promising non-invasive technology with the potential to manipulate biological functions deep inside tissues with high spatiotemporal accuracy and limited off-target effects.
Focused ultrasound stimulation consists of delivering acoustic energy at precise locations deep inside living organisms. Depending on acoustic pulse parameters, this energy can have a variety of medical uses. For instance, the Food and Drug Administration has approved the use of High-intensity Focused Ultrasound (HiFU) for thermal ablation of prostate tumors, tremor-causing brain regions, uterine fibroids and pain-causing nerve endings in bone metastases1. HiFu-mediated microbubble cavitation is also used to transiently open the blood-brain barrier for the targeted delivery of systemically-administered therapeutics2. The spatial-peak pulse-average intensity (Isppa) and spatial-peak temporal-average intensity (Ispta) used for HiFU applications are typically above several kW cm-2 and produce pulse pressure of several tens of MPa. These intensity values are far above the FDA-approved Isppa and Ispta limits for diagnostic ultrasound, 190 W cm-2 and 720 mW cm-2, respectively3. In contrast, recent studies have shown that non-destructive pulsed ultrasound stimulation that are within or near the range of diagnostic ultrasound intensity limits (LIPUS) can be effective to remotely and safely manipulate neural firing4,5,6,7,8, hormonal secretion9,10 and bioengineered cells11. Yet, the cellular and molecular mechanisms by which cells sense and respond to ultrasound remain unclear, precluding clinical translation of LIPUS. Hence, in the past few years, studies of artificial membranes, cultured cells and animals stimulated with ultrasound have gained momentum to reveal biophysical and physiological processes modulated by LIPUS12,13,14,15.
Sound consists of a vibration propagating through a physical medium. An ultrasound is a sound with a frequency above the human audible range (i.e., above 20 kHz). In a laboratory setting, ultrasound waves are generally produced by piezoelectric transducers that contain a material that vibrates in response to an electrical field oscillating in a specific high-frequency bandwidth. Two types of transducers exist: single element transducers and transducer arrays. Single element piezoelectric transducers possess a curved surface which acts as a focusing lens and hence concentrates acoustic energy into a defined region called the focal zone. Single element transducers are much cheaper and easier to operate than transducer arrays. This article will focus on single element transducers.
The size of the focal zone of a focused single element transducer depends on the geometric properties of the acoustic lens and on its acoustic frequency. To achieve a millimeter-size focal zone with a single element transducer, ultrasound frequencies in the MHz range are generally required. Unfortunately, acoustic waves at such frequency are very rapidly attenuated when propagated in a tenuous medium such as air. Thus, MHz ultrasound waves need to be generated and propagated to the sample in a denser material such as water. This constitutes the first challenge in integrating LIPUS modality to a microscope.
A second challenge is to minimize physical interfaces between materials with different acoustic impedances (which is a product of material density and the acoustic velocity) along the acoustic path. These interfaces can reflect, refract, scatter and absorb acoustic waves, making it difficult to quantify the amount of acoustic energy effectively delivered to a sample. They may also create unwanted mechanical artefacts. For instance, reflections produced perpendicular to acoustic mismatch impedance interfaces create backpropagating waves that interfere with forward-propagating ones. Along the interference path, the waves cancel each other at fixed regions of spaces called nodes and sum up at alternating regions called anti-nodes, creating so-called standing waves (Figure 1). It is important for the experimentalist to be able to control or eliminate these experimental interfaces in vitro as they may not exist in vivo.
Fluorescence measurement of optical reporters is a well-known method to interrogate transparent biological samples in real-time and with no physical disturbance. This approach is thus ideal for LIPUS studies as any physical probes present in the sonicated area will introduce mechanical artefacts. This protocol describes the implementation and operation of LIPUS to a commercial epi-fluorescence microscope.
Protocol
1. Growing Cells on Acoustically-Transparent Polyester Film
- Drill a 12 mm hole size at the bottom of a standard 35 mm culture dish using a vertical press-drill. Move the drill slowly and wear eye protection. Remove pieces of plastic attached to the bottom of the dish using a blade to create a smooth surface on the external side (Figure 2).
- Apply a thin layer of marine-grade epoxy or glue at the external bottom surface of the dish.
- Place a film of polyester (2.5 µm thickness) against the external bottom surface of the dish and press firmly to make sure the epoxy/glue spreads evenly between the film and the thick plastic surface. Gently pull the film in a centrifugal manner with fingers to create a flat surface (Figure 2).
- When the epoxy/glue has dried, briefly rinse-dry the polyester-bottom dish with 95% ethanol and sterilize by placing the dish and the inside surface of its lid under a strong 254 nm UV excitation source. Adjust duration and intensity to deliver a UV dose of approximately 330 mJ cm-2 for complete destruction of most types of micro-organisms. This energy approximately corresponds to a duration of 5 min using a 1,000 µW cm-2 UV illumination.
- Aliquot commercially available extracellular matrix protein mixtures (EMPM) in small tubes (50-100 µL) and store them at -20 °C or less in sterile conditions.
- In a sterile environment (e.g., inside a biosafety cabinet), dilute a frozen stock of EMPM with a desired culture medium to 1:100. Work on ice to prevent EMPM polymerization at room temperature. Quickly apply 100 µL of the medium mixture onto the polyester film. Place the lid back on the dish to maintain sterility.
- Incubate EMPM-coated polyester bottom dishes in a cell culture CO2 incubator at 37 °C for 6-12 h.
- After incubation, aspirate the excess medium and directly seed the surface with cells at the desired density. Work under sterile condition to maintain sterility.
2. LIPUS Implementation
- Place a water tank underneath the objective of an upright microscope with large working volume and without illumination hardware in the transmission path.
- Using commercially-available optomechanical components, place a sample holder below the objective and a transducer holder underneath the sample holder. For subsequent sample search and ultrasound alignment, mount these two holders on translation stages.
- Place the moving parts and actuators of translation stages either outside the tank or above the water line to avoid water damage. Only use non-corrosive materials such as anodized aluminum or stainless steel for immersed optomechanical components.
- Fill the tank with deionized and degassed water before utilizing the immersion transducer. The water line should coincide with the horizontal plane of the sample holder (Figure 3).
NOTE: Deionized water prevents electrical coupling in presence of high electric fields. Degassing will also prevent scattering and alterations of acoustic waves. Drain water after each experiment using a pump or valve so that the water line falls below the position of the transducer. Also, replace or filter water frequently and clean-up the water tank as needed to avoid growth of microorganisms.
3. Oblique Acoustic Excitation
- Using commercially available optomechanical components, orient the transducer in an oblique position with respect to the optical path. This will ensure that any reflected waves will be directed away from the sample (Figure 3 and Figure 4).
4. Driving the Transducer
NOTE: Ultrasound transducers convert oscillating electrical energy into mechanical expansion/contraction of a piezoelectric material. This conversion produces energy loss in the form of heat energy. Hence, while transducers do possess a peak input voltage limit, they also possess an electrical power limit to avoid thermal damage to the piezoelectric element:
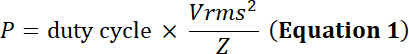
with the duty cycle the relative fraction of time of electrical simulation, P the electrical power (in Watts), Vrms the input root-mean-square voltage (in Volts) of the alternative voltage source and Z the electrical impedance (in Ohms).
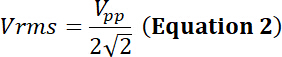
with Vpp the peak-to-peak input voltage applied to the transducer.
- Create a sinusoidal wave form containing the desired frequency, number of cycles per pulse, and pulse repetition frequency using a commercial function generator. However, the relatively high Vpp needed to effectively drive standard ultrasound transducers often requires the addition of a power amplifier to amplify the output (i.e., increase the amplitude of Vpp) of the function generator.
NOTE: For example, a transducer’s manufacturer indicates the power limit for a given transducer is 35 W. Will a sinusoidal peak-to-peak input voltage (Vin) of 500 mV at a duty cycle of 50% and amplified through a 50 dB/100 W amplifier be within the power limit of this transducer?- To answer this question, calculate the voltage after amplification. For a radio-frequency (RF) power amplifier, the amplification factor (dB) is defined by:
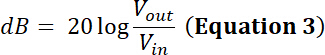
Thus, the amplified voltage has an amplitude output Vpp (Vpp = Vout) of: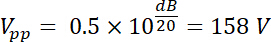
Using Equations 1 and 2, and using 50 Ω as electrical impedance, the corresponding power generated by this voltage is:
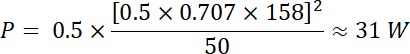
This stimulation is therefore within the power limit of the transducer. - Using the example above, calculate the waveform parameters (Vpp, frequency, pulse duration and pulse repetition frequency) that correspond to the power and voltage limits provided by the transducer’s manufacturer. Make sure to respect these limits to avoid damaging the transducer and other connected instruments.
- To answer this question, calculate the voltage after amplification. For a radio-frequency (RF) power amplifier, the amplification factor (dB) is defined by:
- Choose a function generator that operates within a frequency range compatible with the ultrasound transducer. Adjust the frequency of the function generator to the nominal peak frequency of the transducer.
- Create a sinusoidal voltage pulse of the desired duration and repetition frequency using the burst mode of the function generator. Adjust peak-to-peak voltage to a desired value. Make sure that the pulse duration is shorter than the elapsed time between two consecutive pulses.
- Check that the waveform corresponds to the desired signal by connecting the output of the function generator to the input of an oscilloscope.
- Connect the output of the function generator to the input of a power RF amplifier (Figure 4). Make sure that the stimulation parameters are within the limits of the transducer’s manufacturer.
5. Beam Alignment
- Choose a hydrophone that operates with a frequency range and acoustic intensity compatible with the frequency and intensity of the ultrasound transducer.
- Carefully bring the tip of a hydrophone probe into focus within the objective field of view at the position corresponding to the position of the sample (Figure 4).
- Make sure that both probe and transducer are immersed in deionized and degassed water. Do not bump the tip of the hydrophone with any physical object other than water as this will alter its coating and affect the measurement.
- Perform a gross pre-alignment of the transducer by visually positioning its acoustic axis toward the hydrophone probe. Makes sure that the distance between the transducer’s surface and the hydrophone tip correspond approximately to the transducer’s focal length.
- Connect the hydrophone output to one of the oscilloscope’s signal input. Connect the synchronization trigger from the function generator to another oscilloscope input. Visualize both signals simultaneously on the oscilloscope.
- Drive the transducer with few ultrasound cycles at a low duty cycle and low amplitude to avoid damaging the probe. Check with the hydrophone’s manufacturer safe operation conditions to avoid damaging the hydrophone tip.
- Adjust the s/division knob according to the travel time of ultrasound from the transducer’s surface to the hydrophone. Look for a hydrophone signal on the oscilloscope after the synchronization trigger.
- Slowly actuate the transducer using a motorized or manual XYZ stage. Leave the transducer into the position that correlates with the maximal hydrophone signal (Figure 4).
NOTE: If no signal is detected it is possible that the intensity of the acoustic pulses is too low or that the beam is mis-aligned or scattered by an object. Check regularly that the hydrophone and transducer are visually pre-aligned and that no bubbles or physical object are present in the path except the polyester film. If no signal is still detected, increase the input voltage by a small amount to increase the amplitude of hydrophone signal.
6. Determination of Ultrasound Pulse Pressure and Intensity
- With the beam aligned, measure the peak-to-peak amplitude of the hydrophone output at the oscilloscope for various voltages driving the transducer. Make sure not to exceed the pressure limit recommended by the hydrophone’s manufacturer.
- Convert these measurements into pressure and/or acoustic intensity values using the calibration method provided by the hydrophone’s manufacturer.
NOTE: The acoustic intensity can be determined from the pressure and vice versa using the formula:

with I the acoustic pressure (in W m-2), P the acoustic pressure (in Pa), ρ the density of propagating material (1,000 kg m-3 for water) and c the speed of sound in propagating medium (for water, c = 1,500 m s-1). - Create calibration curves using these measurements.
NOTE: The pressure vs. voltage and intensity vs. voltage curves have a linear and parabolic shape, respectively. - Determine the pressure and/or intensity value of a desired driving voltage by using the corresponding calibration curve.
7. Calcium-Sensitive/LIPUS Live-Cell Fluorescence Imaging
- Replace the cell’s culture medium with a desired imaging buffer containing 5 µM of a cell-permeant calcium-sensitive dye (e.g., Fluo-4 AM). Incubate the culture dish in a CO2 incubator at 37 °C for 1 h.
- Carefully wash cells with the same buffer free of dye.
- Place the dish in the sample holder. Excite the cells using blue light illumination (490 nm) and adjust excitation intensity and camera exposure to avoid excessive bleaching or pixel saturation.
- Perform time-lapse imaging using desired image acquisition settings. Use an immersion objective for better image quality and with long working distance to reduce undesired reflections (see Figure 4).
Results
Figure 5 is an example of LIPUS experiment multiplexed with calcium imaging. Glioblastoma cells (A-172) were grown on EMPM coated polyester film in standard culture medium (supplemented with 10% serum and 1% antibiotics) and incubated with the calcium-sensitive fluorescent reporter Fluo-4 AM. Cells were imaged using a 10X immersion lens and illuminated with a white LED light source and fluorescence light was collected using a standard GFP filter set. LIPUS wa...
Discussion
A main advantage of focused ultrasound is its ability to non-invasively deliver mechanical and/or thermal energy to biological samples with high spatio-temporal precision. Other techniques intended to mechanically stimulate cells usually employ invasive physical probes (e.g., cell-poking) or requires the interaction of high energy laser beams with foreign objects (e.g., optical tweezers). Magnetic heating can heat specific spatial locations inside biological samples but requires the presence of foreign ...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Drs. Mikhail Shapiro and Nikita Reznik for fruitful discussions. This work was supported by start-up funds from Western University of Health Sciences and NIH grant R21NS101384.
Materials
| Name | Company | Catalog Number | Comments |
| upright microscope with large working volume | Thorlabs | CERNA | |
| upright microscope with large working volume | Scientifica | SliceScope | |
| optomechanical components | Thorlabs | n/a | |
| needle hydrophone | ONDA Corporation | HNP/C/R/A/T series + AH/G pre-amplifier | |
| needle hydrophone | Precision Acoustics | n/a | |
| fiber optic hydrophone | ONDA Corporation | HFO series | |
| fiber optic hydrophone | Precision Acoustics | n/a | |
| oscilloscope | Keysight Technology | DSOX2004A (4-channels 70MHz) | |
| function generator | Keysight Technology | 33500B (20MHz single-channel) | |
| RF power amplifier | Electronic Navigation Industries (ENI) | 325LA, 525LA, 240L, 350L, A075, 2100L, 3100LA | |
| RF power amplifier | Electronics & Innovation (E&I) | ||
| immersion ultrasound transducer | Olympus | focused immersion transdcuers | |
| immersion ultrasound transducer | Benthowave Instrument | HiFu transducer BII-76 series | |
| immersion ultrasound transducer | Precision Acoustics | Piezo-ceramic or HiFu transducers | |
| immersion ultrasound transducer | Ultrasonic-S-lab | HiFu transducers made to order | |
| high-density Matrigel | Corning | VWR 80094-330 | |
| Mylar film 2.5 microns | Chemplex | CAT.NO:107 |
References
- Elhelf, I. A. S., et al. High intensity focused ultrasound: The fundamentals, clinical applications and research trends. Diagnostic and Interventional Imaging. 99 (6), 349-359 (2018).
- Toccaceli, G., Delfini, R., Colonnese, C., Raco, A., Peschillo, S. . Emerging strategies and future perspective in neuro-oncology using Transcranial Focused Ultrasound Technology. , (2018).
- Duck, F. A. Medical and non-medical protection standards for ultrasound and infrasound. Progress in Biophysics and Molecular Biology. 93 (1-3), 176-191 (2007).
- Legon, W., et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nature Neuroscience. 17 (2), 322-329 (2014).
- Tyler, W. J. The mechanobiology of brain function. Nature Reviews: Neuroscience. 13 (12), 867-878 (2012).
- Tyler, W. J. Noninvasive neuromodulation with ultrasound? A continuum mechanics hypothesis. Neuroscientist. 17 (1), 25-36 (2011).
- Tufail, Y., et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 66 (5), 681-694 (2010).
- Tyler, W. J., et al. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PloS One. 3 (10), e3511 (2008).
- Suarez Castellanos, I., et al. Calcium-dependent ultrasound stimulation of secretory events from pancreatic beta cells. Journal of Therapeutic Ultrasound. 5, 30 (2017).
- Suarez Castellanos, I., Jeremic, A., Cohen, J., Zderic, V. Ultrasound Stimulation of Insulin Release from Pancreatic Beta Cells as a Potential Novel Treatment for Type 2 Diabetes. Ultrasound in Medicine and Biology. 43 (6), 1210-1222 (2017).
- Ibsen, S., Tong, A., Schutt, C., Esener, S., Chalasani, S. H. Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nature Communications. 6, 8264 (2015).
- Prieto, M. L., Firouzi, K., Khuri-Yakub, B. T., Maduke, M. Activation of Piezo1 but Not NaV1.2 Channels by Ultrasound at 43 MHz. Ultrasound in Medicine and Biology. 44 (6), 1217-1232 (2018).
- Kubanek, J., et al. Ultrasound modulates ion channel currents. Scientific Reports. 6, 24170 (2016).
- Prieto, M. L., Omer, O., Khuri-Yakub, B. T., Maduke, M. C. Dynamic response of model lipid membranes to ultrasonic radiation force. PloS One. 8 (10), e77115 (2013).
- Sato, T., Shapiro, M. G., Tsao, D. Y. Ultrasonic Neuromodulation Causes Widespread Cortical Activation via an Indirect Auditory Mechanism. Neuron. 98 (5), 1031-1041 (2018).
- O'Brien, W. D. Ultrasound-biophysics mechanisms. Progress in Biophysics and Molecular Biology. 93 (1-3), 212-255 (2007).
- Shapiro, M. G., Homma, K., Villarreal, S., Richter, C. P., Bezanilla, F. Corrigendum: Infrared light excites cells by changing their electrical capacitance. Nature Communications. 8, 16148 (2017).
- Shapiro, M. G., Homma, K., Villarreal, S., Richter, C. P., Bezanilla, F. Infrared light excites cells by changing their electrical capacitance. Nature Communications. 3, 736 (2012).
- Shapiro, M. G., Priest, M. F., Siegel, P. H., Bezanilla, F. Thermal mechanisms of millimeter wave stimulation of excitable cells. Biophysical Journal. 104 (12), 2622-2628 (2013).
- Hwang, J. Y., et al. Investigating contactless high frequency ultrasound microbeam stimulation for determination of invasion potential of breast cancer cells. Biotechnology and Bioengineering. 110 (10), 2697-2705 (2013).
- Nakano, M., et al. Genetically encoded ratiometric fluorescent thermometer with wide range and rapid response. PloS One. 12 (2), e0172344 (2017).
- Donner, J. S., Thompson, S. A., Kreuzer, M. P., Baffou, G., Quidant, R. Mapping intracellular temperature using green fluorescent protein. Nano Letters. 12 (4), 2107-2111 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved