A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Microfluidic Production of Lysolipid-Containing Temperature-Sensitive Liposomes
In This Article
Summary
The protocol presents the optimized parameters for preparing thermosensitive liposomes using the staggered herringbone micromixer microfluidics device. This also allows co-encapsulation of doxorubicin and indocyanine green into the liposomes and the photothermal-triggered release of doxorubicin for controlled/triggered drug release.
Abstract
The presented protocol enables a high-throughput continuous preparation of low temperature-sensitive liposomes (LTSLs), which are capable of loading chemotherapeutic drugs, such as doxorubicin (DOX). To achieve this, an ethanolic lipid mixture and ammonium sulfate solution are injected into a staggered herringbone micromixer (SHM) microfluidic device. The solutions are rapidly mixed by the SHM, providing a homogeneous solvent environment for liposomes self-assembly. Collected liposomes are first annealed, then dialyzed to remove residual ethanol. An ammonium sulfate pH-gradient is established through buffer exchange of the external solution by using size exclusion chromatography. DOX is then remotely loaded into the liposomes with high encapsulation efficiency (> 80%). The liposomes obtained are homogenous in size with Z-average diameter of 100 nm. They are capable of temperature-triggered burst release of encapsulated DOX in the presence of mild hyperthermia (42 °C). Indocyanine green (ICG) can also be co-loaded into the liposomes for near-infrared laser-triggered DOX release. The microfluidic approach ensures high-throughput, reproducible and scalable preparation of LTSLs.
Introduction
LTSL formulation is a clinically relevant liposomal product that has been developed to deliver the chemotherapeutic drug doxorubicin (DOX) and allows efficient burst drug release at clinically attainable mild hyperthermia (T ≈ 41 °C)1. The LTSL formulation consists of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), the lysolipid 1-stearoyl-2-hydroxy-sn-glycero-3-phosphatidylcholine (MSPC; M stands for "mono") and PEGylated lipid 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000). Upon reaching the phase transition temperature (Tm ≈ 41 °C), the lysolipid and DSPE-PEG2000 together facilitate the formation of membrane pores, resulting in a burst release of the drug2. The preparation of LTSLs primarily uses a bulk top-down approach, namely lipid film hydration and extrusion. It remains challenging to reproducibly prepare large batches with identical properties and in sufficient quantities for clinical applications3.
Microfluidics is an emerging technique for preparing liposomes, offering tunable nanoparticle size, reproducibility, and scalability3. Once the manufacturing parameters are optimized, the throughput could be scaled-up by parallelization, with properties identical to those prepared at bench scale3,4,5. A major advantage of microfluidics over conventional bulk techniques is the ability to handle small liquid volumes with high controllability in space and time through miniaturization, allowing faster optimization, while operating in a continuous and automated manner6. Production of liposomes with microfluidic devices is achieved by a bottom-up nanoprecipitation approach, which is more time and energy efficient because homogenization processes such as extrusion and sonication are unnecessary7. Typically, an organic solution (e.g. ethanol) of lipids (and hydrophobic payload) is mixed with a miscible non-solvent (e.g. water and hydrophilic payload). As the organic solvent mixes with the non-solvent, the solubility for the lipids is reduced. The lipid concentration eventually reaches a critical concentration at which the precipitation process is triggered7. Nanoprecipitates of lipids eventually grow in size and close into a liposome. The main factors governing the size and homogeneity of the liposomes are the ratio between the non-solvent and solvent (i.e. aqueous-to-organic flow rate ratio; FRR) and the homogeneity of the solvent environment during the self-assembly of lipids into liposomes8.
Efficient fluid mixing in microfluidics is therefore essential to the preparation of homogeneous liposomes, and various designs of mixers have been employed in different applications9. Staggered herringbone micromixer (SHM) represents one of the new generations of passive mixers, which enables high throughput (in range of mL/min) with a low dilution factor. This is superior to traditional microfluidic hydrodynamic mixing devices8,10. The SHM has patterned herringbone grooves, which rapidly mix fluids by chaotic advection9,11. The short mixing timescale of SHM (< 5 ms, less than the typical aggregation time scale of 10–100 ms) allows lipid self-assembly to occur in a homogenous solvent environment, producing nanoparticles with uniform size distribution3,12.
The preparation of LTSLs with microfluidics is, however, not as straightforward compared to conventional liposomal formulations due to the lack of cholesterol8, without which lipid bilayers are susceptible to ethanol-induced interdigitation13,14,15. Until now, the effect of residual ethanol presents during the microfluidic production of liposomes has not been well understood. The majority of the reported formulations are inherently resistant to interdigitation (containing cholesterol or unsaturated lipids)16, which unlike LTSLs are both saturated and cholesterol-free.
The protocol presented herein uses SHM to prepare LTSLs for temperature triggered-release drug delivery. In the presented method, we ensured the microfluidic-prepared LTSLs are nano-sized (100 nm) and uniform (dispersity < 0.2) by dynamic light scattering (DLS). Furthermore, we encapsulated DOX using the transmembrane ammonium sulfate gradient method (also known as remote loading)17 as a validation of the integrity of the LTSL lipid bilayer. Remote loading of DOX requires the liposome to maintain a pH-gradient in order to achieve high encapsulation efficiency (EE), which is unlikely to happen without an intact lipid bilayer. In this presented method, distinctive from typical microfluidic liposome preparation protocols, an annealing step is required before the ethanol is removed to enable the remote loading capability; i.e. to restore the integrity of the lipid bilayer.
As mentioned previously, hydrophilic and hydrophobic payloads can also be introduced to the initial solutions for the simultaneous encapsulation of payloads during the formation of LTSLs. As a proof-of-concept, indocyanine green (ICG), an FDA-approved near-infrared fluorescent dye, which is also a promising photothermal agent, is introduced to the initial lipid mixture and successfully co-loaded into the LTSLs. An 808 nm laser is used to irradiate the DOX/ICG-loaded LTSLs and successfully induce photothermal heating-triggered burst release of DOX within 5 min.
All the instruments and materials are commercially available, ready-to-use, and without the need for customization. Since all the parameters for formulating LTSLs have been optimized, following this protocol, researchers with no prior knowledge of microfluidics could also prepare the LTSLs, which serves as the basis of a thermosensitive drug delivery system.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Equipment setup
- Assemble the syringe pumps and SHM as follows.
- Connect the "To Computer" port of the secondary syringe pump (Pump 02, for aqueous solution) to the "To Network" port of the master syringe pump (Pump 01, for ethanol lipid solution) using Pump to Pump network cable (Figure 1, yellow).
- Connect the "To Computer" port of the master pump to the "RS232 Serial" port of the computer using PC to Pump network cable (Figure 1, blue).
- Connect tubing to each of the inlets and outlets of the SHM using a nut and ferrule. Convert the terminal of the tubing for both inlets to female Luer using another nut and ferrule and a union assembly. Longer tubing of the inlets allows easier attachment to the syringes (Figure 2).
- Set up the pump control software.
- Assign the address of the master syringe pump and secondary syringe pump to "Ad:01" and "Ad:02", respectively, using the "Setup" button of the syringe pump. This only needs to be done for the first time.
- Open the pump control software on the computer. The two syringe pumps should be detected automatically, followed by a beeping sound. Otherwise, click Pumps and Search for pumps to update the connection. (Figure 3).
- Assign Diameter to 12.45 (mm) by choosing "HSW Norm-Ject 5 cc (Dia=12.45)".
- Assign Rate to 0.25 mL/min for Pump 01 (ethanol lipid solution) and 0.75 mL/min for Pump 02 (aqueous solution). The flow rates correspond to a total flow rate (TFR) of 1 mL/min and aqueous-to-ethanol flow rate ratio (FRR) of 3.
- Assign Volume to any values above 5 mL.
NOTE: The targeted infusion volume is set greater than the loaded liquid volume considering the void volume of the tubing. - Select INF (infusion) mode for both pumps.
- Press Set to confirm the settings.
2. Prepare the LTSLs
- Prepare a LTSL10 or LTSL10-ICG lipid mixture (see Table 1).
- Withdraw 1 mL of lipid mixture and at least 3 mL of (NH4)2SO4 solution using two 5 mL Luer lock syringes.
- Install the two syringes onto the syringe pumps in the upright position by sliding the barrel flange of the syringe to the syringe retainer of the pump, and the plunger flange of the syringe to the pusher block of the pump (Figure 4).
- Wrap the end of the heating tape to the syringe with the aqueous solution. Wrap the other end of the heating tape and temperature probe of the thermostat around the syringe with the lipid solution. It is helpful to practice this step with empty syringes in place in order to ease the assembly process (Figure 5A).
- Connect the two syringes to the female Luer adaptors of the corresponding inlets of the SHM. Make sure the syringes containing the lipid mixture and (NH4)2SO4 solutions are connected ethanol inlet and aqueous inlet, respectively. Adjust the plunger position to remove air bubbles from the syringes (Figure 5B).
NOTE: Ensure the syringes are still securely positioned onto the syringe retainer of the pumps. - Heat up the syringes to above 51 °C using the heating tape using a 10 s heating session. Allow the thermostat to update the temperature of the syringes. Repeat this step in the following steps to maintain the temperature during the infusion.
CAUTION: Turn off the heating tape after 10 s to prevent temperature overshoot and allow the thermostat to update the actual temperature. The heating tape should also be handled with care as its temperature rises very quickly. Heating continuously may damage the equipment and syringes, due to the time delay of the thermostat for updating the measured temperature. - Once the temperature is above 51 °C, run the syringe pumps by pressing Run All in the pump control software (Figure 3).
- Ensure the fluid flow is free of air bubbles and any leakage. Dispose the initial volume (around 0.5 mL) of liquid from the outlet as waste.
NOTE: This initial waste volume is not definite and depends on the internal volume of the setup, which is the volume for fluid to travel from the syringes through the tubing and SHM to the outlet. - Collect the rest of the liquid as liposome samples into a microcentrifuge tube or bijou vial.
- Pause/stop the infusion when the liquid in either of the syringes are almost empty.
NOTE: The pumps should be stopped manually, since the pumps may not accurately detect the position when the syringes are empty. - Place the collected liposome solutions in a 60 °C water bath to anneal for 1.5 h.
NOTE: This step is essential in enabling drug loading into the liposomes. - Transfer the solutions to dialysis tubes. Dialyze the solutions against 1 L of 240 mM (NH4)2SO4 at 37 °C for at least 4 h to obtain purified liposomes.
NOTE: The protocol can be paused here. Liposomes at this step are at 5 mM of phospholipid. Purified liposomes can be stored at 4 °C. - To clean the SHM for repeated use, flush the SHM sequentially with deionized water, ethanol and dry with nitrogen gas.
3. Remote loading of DOX into LTSLs by transmembrane pH gradient
- Exchange external buffer of LTSLs to HEPES-buffered saline (HBS) by using size exclusion chromatography (SEC) to establish a transmembrane pH gradient.
- Add a total of 25 mL of HBS to the top of a SEC column to prepare the column. Allow all eluent to elute through the column and dispose the eluate.
- Add 1 mL of dialyzed liposomes, prepared from step 2.12, to the column and dispose the elute.
- Add 1.5 mL of HBS to the column and dispose the elute.
- Add 3 mL of HBS to the column and collect the 3 mL of elute.
NOTE: The protocol can be paused here. Liposomes are collected at this step and are at 1.67 mM of phospholipid. Buffer exchanged liposomes can be stored at 4 °C.
- Incubate LTSLs with doxorubicin (DOX) and purify LTSLs.
- Add DOX solution in 1:20 DOX-to-phospholipid molar ratio into 1 mL of buffer-exchanged liposomes solution (1.67 mmol) contained in a bijou vial. This can be achieved by adding 48.4 µL of 1 mg/mL DOX solution (83.4 µmol).
- Place the bijou vial in a 37 °C water bath for 1.5 h to allow DOX loading into the liposomes.
- Mix 10 µL of the liposomes with 170 µL of HBS and 20 µL of 1% (v/v) Triton X-100 solution in a black 96-well plate. Repeat for three wells. These wells correspond to the "before purification" DOX content.
- In case of preparing LTSL10-ICG, mix 40 µL of the liposomes with 160 µL of DMSO in a clear 96-well plate. Repeat for three wells. These wells correspond to the "before purification" ICG content.
- Purify the liposome solution as described in step 3.1.
NOTE: To reuse the column for future purification, clean the column from free DOX by first adding 1 mL of diluted 0.5 M NaOH solution before performing step 3.1.1. Free DOX in red will turn violet-blue and elute through the column quickly. - Mix 30 µL of the purified liposomes solution with 150 µL of HBS and 20 µL of 1% (v/v) Triton X-100 solution in a black 96-well plate. Repeat for three wells. These wells correspond to the "after purification" DOX content.
- In case of LTSL10-ICG, mix 40 µL of the purified liposomes solution with 160 µL of DMSO in a clear 96-well plate. Repeat for three wells. These wells correspond to the "after purification" ICG content.
- Measure the DOX fluorescence intensity of the wells before (step 3.2.3) and after (step 3.2.5) purification, using a microplate reader (λex = 485 nm, λem = 590 nm).
- Calculate the encapsulation efficiency of DOX (DOX EE) by taking the ratio of the fluorescence intensities before and after purification.
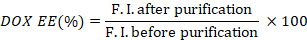
- Measure the ICG absorbance of the wells before and after purification, using a microplate reader (600 to 1000 nm).
- Calculate the encapsulation efficiency of ICG (ICG EE) by taking the ratio of the absorbance at 792 nm before and after purification, taking into account the dilution factor (3 times) during the purification.

4. Dynamic Light Scattering (DLS)
- Add 50 µL of liposomes solution (step 2.12) to 450 µL of deionized water.
- Place the cuvette inside the DLS instrument and perform the measurement according to the manufacturer's instructions.
- Record the mean Z-average diameter and dispersity of three measurements for each sample.
5. Differential scanning calorimetry (DSC)
- Concentrate 1 mL of the liposomes samples (step 2.12) with a centrifugal filter unit to 0.5 mL (final lipid concentration of 10 mM). Using a fixed-angle rotor, spin at 7500 x g for approximately 15 min.
- Transfer 20 µL of (NH4)2SO4 solution and liposomes samples to two respective DSC pans. Seal the pans with DSC hermetic lids using the DSC sample press kit.
- Measure the sample from 30 °C to 60 °C at a heating rate of 1 °C/min using a differential scanning calorimeter.
- Analyze the data with appropriate software. Take the phase transition temperature (Tm) as the onset of the phase transition (melting peak), which is measured by the x-intercept of the tangent of the point of maximum slope.
6. Doxorubicin release
- Preheat HBS at designated temperature (37 or 42 °C) using a water bath. Prepare an ice water bath for quenching the samples.
- Add 100 µL of purified DOX-loaded liposomes (step 3.2.5) into 1.9 mL of HBS in a microcentrifuge tube. Place the tube into the water bath of the designated temperature.
- Withdraw immediately 200 µL of samples from the tube and quickly place it in the ice water bath to quench any subsequent drug release. This sample corresponds to the initial (t = 0) time point.
- Withdraw 200 µL of samples at subsequent time points (t = 5, 10, 15, 30, 60 min) and quickly place it in the ice water bath to quench any drug release.
- Mix 50 µL of sample of each time point with 150 µL of HBS in a black 96-well plate. Measure the DOX fluorescence intensity using a plate reader.
- Add 20 µL of 1% (v/v) Triton X-100 into random selected wells prepared in step 6.5. Measure the DOX fluorescence intensity of these wells using a plate reader. These values correspond to the fully released (t = ∞; 100% release) time point.
- Calculate and plot the percentage of DOX released by interpolating the fluorescence intensity of each time points (I(t)), compared to the initial (I(0)), and fully released (I(∞)), value.
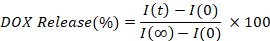
7. Laser Heating and Triggered Release
- Set water bath temperature to 37 °C and allow the temperature to stabilize.
- Add 200 µL of DOX loaded LTSL10-ICG ([ICG] = 10 µg/mL) to a clear 96-well plate, then place it in the water bath, keep the bottom immersed in water.
- Set the current of the laser system to 2.27 A. Place the collimator of the laser system at 5 cm vertically above the surface of the 96-well plate, which corresponds to an energy flux of 0.5 W/cm2 [Figure 6].
CAUTION: The laser system should be operated in compliance with relevant laser safety measures. - Switch on the laser and monitor the temperature every minute using a fiber optic temperature probe.
- At 5 and 10 min, withdraw 10 µL of laser-irradiated liposomes from the clear 96-well plate at and mix with 190 µL of HBS for three wells in a black 96-well plate.
- Mix 10 µL of the liposomes with 170 µL of HBS and 20 µL of 1% (v/v) Triton X-100 solution for three wells in a black 96-well plate. These wells correspond to the "100% released" DOX content. Measure the DOX fluorescent intensity and calculate the DOX release as described in step 6.7.
Access restricted. Please log in or start a trial to view this content.
Results
The preparation of LTSLs by microfluidics requires the lipid composition of DPPC/MSPC/DSPE-PEG2000 (80/10/10, molar ratio; LTSL10). Figure 7A (left) shows the appearance of as-prepared LTSL10 from step 2.9, as a clear and non-viscous liquid. LTSL10 formulation is developed from the conventional formulation, LTSL4 (DPPC/MSPC/DSPE-PEG2000, 86/10/4, molar ratio) since LTSL4 forms a gel-like viscous sample, as indicated by t...
Access restricted. Please log in or start a trial to view this content.
Discussion
The presented protocol describes the preparation of low temperature-sensitive liposomes (LTSLs) using a staggered herringbone micromixer (SHM). The LTSL10 formulation enables temperature-triggered burst release of doxorubicin within 5 minutes at a clinically attainable hyperthermic temperature of 42 °C. Indocyanine green (ICG) can also be co-loaded for photothermal heating triggered the release of DOX. The method relies on: (i) self-assembly of phospholipids into liposomes under a homogenized solvent environment pro...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Prostate Cancer UK (CDF-12-002 Fellowship), and the Engineering and Physical Sciences Research Council (EPSRC) (EP/M008657/1) for funding.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) | Lipoid | PC 16:0/16:0 (DPPC) | |
| 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) | Lipoid | PE 18:0/18:0-PEG 2000 (MPEG 2000-DSPE) | |
| 1-stearoyl-2-hydroxy-sn-glycero-3-phosphocholine (MSPC) | Avanti Polar Lipid | 855775P-500MG | Distributed by Sigma-Adrich; also known as Lyso 16:0 PC (Not to be confused with 14:0/18:0 PC, which is also termed MSPC) |
| 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) | Sigma-Aldrich | H3375-100G | |
| Adapters, Female Luer Lock to 1/4"-28UNF | IDEX Health & Science | P-624 | Requires 2 units. For the inlets |
| Adapters, Union Assembly, 1/4"-28UNF | IDEX Health & Science | P-630 | Requires 2 units. (One unit included 2 nuts and 2 ferrules) |
| Ammonium Sulfate ((NH4)2SO4) | Sigma-Aldrich | 31119-1KG-M | |
| Bijou vial | VWR | 216-0980 | 7 mL, clear, polystyrene vial |
| Centrifugal Filter Unit | Sigma-Aldrich | UFC801008 | 10 kDa MWCO, Amicon Ultra-4 Centrifugal Filter Unit |
| Centrifuge | ThermoFisher Scientific | Heraeus Megafuge 8R | With HIGHConic III Fixed Angle Rotor |
| Cuvette | Fisher Scientific | 11602609 | Disposable polystyrene cuvette, low volume, for DLS measurement |
| Dialysis Kit - Pur-A-Lyzer Maxi | Sigma-Aldrich | PURX12015-1KT | 12-14 kDa MWCO |
| Dimethyl Sulfoxide (DMSO) | Sigma-Aldrich | 34943-1L-M | |
| DLS Instrument | Malvern Panalytical | Zetasizer Nano ZS90 | |
| Doxorubicin Hydrochloride (DOX) | Apollo Scientific | BID0120 | |
| DSC Instrument | TA Instruments | TA Q200 DSC | |
| DSC Tzero Hermetic Lids | TA Instruments | 901684.901 | For DSC measurement |
| DSC Tzero Pans | TA Instruments | 901683.901 | For DSC measurement |
| DSC Tzero Sample Press Kit | TA Instruments | 901600.901 | For DSC measurement |
| Ethanol | VWR | 20821.330 | Absolute, ≥99.8% |
| FC-808 Fibre Coupled Laser System | CNI Optoelectronics Tech | FC-808-8W-181315 | FOC-01-B Fiber Collimator included. |
| Ferrule, 1/4"-28UNF to 1/16" OD | IDEX Health & Science | P-200 | For the outlet |
| Fibre Optic Temperature Probe | Osensa | PRB-G40 | |
| Glass Staggered Herringbone Micromixer (SHM) | Darwin Microfluidics | Herringbone Mixer - Glass Chip | |
| Heating Tape | Omega | DHT052020LD | Can be replaced by other syringe heater such as "HTC" or "SRT series" for slower heating. Manual wiring to a 3-pin plug required for 240V models |
| Indocyanine Green | Adooq | A10473-100 | Distributed by Bioquote Limited (U.K.) |
| Luer-lock Syringe, 5 mL | VWR | 613-2043 | Hanke Sass Wolf SOFT-JECT 3-piece syringes, O.D. 12.45 mm |
| Microplate Reader | BMG Labtech | FLUOstar Omega | Installed with 485 nm (exictation) and 590 nm (emission) filters |
| Microplate, 96-well, Black, Flat-bottom | ThermoFisher Scientific | 611F96BK | For fluorescence measurement in microplate reader |
| Microplate, 96-well, Clear, Flat-bottom | Grenier | 655101 | For absorbance measurement microplate reader |
| Nut, 1/4"-28UNF to 1/16" OD | IDEX Health & Science | P-245 | For the outlet |
| PC to Pump Network Cable for Aladdin, 7ft | World Precision Instruments | NE-PC7 | Optional: Syringe pumps can be operated manually |
| Pump control software - SyringePumpPro Software License for 2 | World Precision Instruments | SYRINGE-PUMP-PRO-02 | Optional: Syringe pumps can be operated manually |
| Pump to Pump Network Cable for Aladdin, 7 ft | World Precision Instruments | NE-NET7 | Optional: Syringe pumps can be operated manually |
| Size exclusion chromatography (SEC) column | GE Life Science | 17085101 | Sephadex G-25 resin in PD-10 Desalting Columns |
| Sodium chloride (NaCl) | Sigma-Aldrich | 31434-1KG-M | |
| Sodium hydroxide (NaOH) | Sigma-Aldrich | S5881-500G | |
| Syringe Pumps & Cable (DUAL-PUMP-NE-1000) | World Precision Instruments | ALADDIN2-220/AL1000-220 | |
| Thermostat Temperature Controller | Inkbird | ITC-308 | Can be replaced by other syringe heater kit/thermostat |
| Triton X-100 | Sigma-Aldrich | X100-100ML | |
| Tubing, ETFE (1/16" OD) | IDEX Health & Science | 1516 | |
| USB To RS-232 Converter | World Precision Instruments | CBL-USB-232 | Optional: For computer without RS-232 port |
| Water Bath | Grant Instruments Ltd. | JB Nova 12 |
References
- Needham, D., Park, J., Wright, A. M., Tong, J. Materials characterization of the low temperature sensitive liposome (LTSL): effects of the lipid composition (lysolipid and DSPE-PEG2000) on the thermal transition and release of doxorubicin. Faraday Discussions. 161, 515-534 (2013).
- Ickenstein, L. M., Arfvidsson, M. C., Needham, D., Mayer, L. D., Edwards, K. Disc formation in cholesterol-free liposomes during phase transition. Biochimica et Biophysica Acta - Biomembranes. 1614 (2), 135-138 (2003).
- Valencia, P. M., Farokhzad, O. C., Karnik, R., Langer, R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nature Nanotechnology. 7 (10), 623-629 (2012).
- Chen, D., et al. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. Journal of the American Chemical Society. 134 (16), 6948-6951 (2012).
- Forbes, N., et al. Rapid and scale-independent microfluidic manufacture of liposomes entrapping protein incorporating in-line purification and at-line size monitoring. International Journal of Pharmaceutics. 556, 68-81 (2019).
- Dittrich, P. S., Manz, A. Lab-on-a-chip: microfluidics in drug discovery. Nature Reviews Drug Discovery. 5 (3), 210-218 (2006).
- Capretto, L., Carugo, D., Mazzitelli, S., Nastruzzi, C., Zhang, X. Microfluidic and lab-on-a-chip preparation routes for organic nanoparticles and vesicular systems for nanomedicine applications. Advanced Drug Delivery Reviews. 65 (11-12), 1496-1532 (2013).
- Cheung, C. C. L., Al-Jamal, W. T. Sterically stabilized liposomes production using staggered herringbone micromixer: Effect of lipid composition and PEG-lipid content. International Journal of Pharmaceutics. 566, 687-696 (2019).
- Suh, Y. K., Kang, S. A. Review on Mixing in Microfluidics. Micromachines. 1 (3), 82-111 (2010).
- Jahn, A., Vreeland, W. N., Gaitan, M., Locascio, L. E. Controlled Vesicle Self-Assembly in Microfluidic Channels with Hydrodynamic Focusing. Journal of the American Chemical Society. 126 (9), 2674-2675 (2004).
- Stroock, A. D. Chaotic Mixer for Microchannels. Science. 295 (5555), 647-651 (2002).
- Belliveau, N. M., et al. Microfluidic Synthesis of Highly Potent Limit-size Lipid Nanoparticles for In Vivo Delivery of siRNA. Molecular Therapy - Nucleic Acids. 1 (8), 37(2012).
- Patra, M., et al. Under the influence of alcohol: The effect of ethanol and methanol on lipid bilayers. Biophysical Journal. 90 (4), 1121-1135 (2006).
- Komatsu, H., Rowe, E. S., Rowe, E. S. Effect of Cholesterol on the Ethanol-Induced Interdigitated Gel Phase in Phosphatidylcholine: Use of Fluorophore Pyrene-Labeled Phosphatidylcholine. Biochemistry. 30 (9), 2463-2470 (1991).
- Lu, J., Hao, Y., Chen, J. Effect of Cholesterol on the in Lysophosphatidylcholine Formation of an Interdigitated Gel Phase and Phosphatidylcholine Binary. Journal of Biochemistry. 129 (6), 891-898 (2001).
- Vanegas, J. M., Contreras, M. F., Faller, R., Longo, M. L. Role of unsaturated lipid and ergosterol in ethanol tolerance of model yeast biomembranes. Biophysical Journal. 102 (3), 507-516 (2012).
- Haran, G., Cohen, R., Bar, L. K., Barenholz, Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1151 (2), 201-215 (1993).
- Sadeghi, N., et al. Influence of cholesterol inclusion on the doxorubicin release characteristics of lysolipid-based thermosensitive liposomes. International Journal of Pharmaceutics. 548 (2), 778-782 (2018).
- Lawaczeck, R., Kainosho, M., Chan, S. I. The formation and annealing of structural defects in lipid bilayer vesicles. Biochimica et Biophysica Acta (BBA) - Biomembranes. 443 (3), 313-330 (1976).
- Komatsu, H., Okada, S. Ethanol-induced aggregation and fusion of small phosphatidylcholine liposome: participation of interdigitated membrane formation in their processes. BBA - Biomembranes. 1235 (2), 270-280 (1995).
- Marsh, D., Bartucci, R., Sportelli, L. Lipid membranes with grafted polymers: physicochemical aspects. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1615 (1-2), 33-59 (2003).
- Hood, R. R., Vreeland, W. N., DeVoe, D. L. Microfluidic remote loading for rapid single-step liposomal drug preparation. Lab on a Chip. 14 (17), 3359-3367 (2014).
- Dalwadi, G., Benson, H. A. E., Chen, Y. Comparison of diafiltration and tangential flow filtration for purification of nanoparticle suspensions. Pharmaceutical Research. , (2005).
- Roces, C., Kastner, E., Stone, P., Lowry, D., Perrie, Y. Rapid Quantification and Validation of Lipid Concentrations within Liposomes. Pharmaceutics. 8 (3), 29(2016).
- Kim, S. -H., Kim, J. W., Kim, D. -H., Han, S. -H., Weitz, D. A. Enhanced-throughput production of polymersomes using a parallelized capillary microfluidic device. Microfluidics and Nanofluidics. 14 (3-4), 509-514 (2013).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved