A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Hydrogen-Deuterium Exchange Mass Spectrometry (HDX-MS) Platform for Investigating Peptide Biosynthetic Enzymes
In This Article
Summary
Lanthipeptide synthetases catalyze multistep reactions during the biosynthesis of peptide natural products. Here, we describe a continuous, bottom-up, hydrogen-deuterium exchange mass spectrometry (HDX-MS) workflow that can be employed to study the conformational dynamics of lanthipeptide synthetases, as well as other similar enzymes involved in peptide natural product biosynthesis.
Abstract
Hydrogen-deuterium exchange mass spectrometry (HDX-MS) is a powerful method for the biophysical characterization of enzyme conformational changes and enzyme-substrate interactions. Among its many benefits, HDX-MS consumes only small amounts of material, can be performed under near native conditions without the need for enzyme/substrate labeling, and can provide spatially resolved information on enzyme conformational dynamics−even for large enzymes and multiprotein complexes. The method is initiated by the dilution of the enzyme of interest into buffer prepared in D2O. This triggers the exchange of protium in peptide bond amides (N-H) with deuterium (N-D). At the desired exchange time points, reaction aliquots are quenched, the enzyme is proteolyzed into peptides, the peptides are separated by ultra-performance liquid chromatography (UPLC), and the change in mass of each peptide (due to the exchange of hydrogen for deuterium) is recorded by MS. The amount of deuterium uptake by each peptide is strongly dependent on the local hydrogen bonding environment of that peptide. Peptides present in very dynamic regions of the enzyme exchange deuterium very rapidly, whereas peptides derived from well-ordered regions undergo exchange much more slowly. In this manner, the HDX rate reports on local enzyme conformational dynamics. Perturbations to deuterium uptake levels in the presence of different ligands can then be used to map ligand binding sites, identify allosteric networks, and to understand the role of conformational dynamics in enzyme function. Here, we illustrate how we have used HDX-MS to better understand the biosynthesis of a type of peptide natural products called lanthipeptides. Lanthipeptides are genetically encoded peptides that are post-translationally modified by large, multifunctional, conformationally dynamic enzymes that are difficult to study with traditional structural biology approaches. HDX-MS provides an ideal and adaptable platform for investigating the mechanistic properties of these types of enzymes.
Introduction
Proteins are structurally dynamic molecules that sample different conformations on time scales ranging from femtosecond-scale bond vibration to rearrangements of entire protein domains which can occur over many seconds1. These conformational fluctuations are often critical aspects of enzyme/protein function. For example, conformational changes induced by ligand binding are often critically important for modulating enzyme function, either by organizing active site residues needed for catalysis, defining substrate binding sites in sequential kinetic mechanisms, shielding reactive intermediates from the environment, or by modulating enzyme function via allosteric networks. Recent studies have also shown that conformational dynamics can be conserved throughout evolution and that perturbations to conserved molecular motions can be correlated with changes in substrate specificity and the emergence of new enzyme functions2,3.
In recent years, hydrogen-deuterium exchange mass spectrometry (HDX-MS) has rapidly emerged as a powerful technique to probe how protein conformational landscapes respond to perturbations such as ligand binding or mutagenesis4,5,6,7. In a typical HDX-MS experiment (Figure 1), a protein of interest is placed into buffer prepared in D2O, which triggers replacement of solvent-exchangeable protons with deuteria. The rate of exchange of the amide moiety of the peptide bonds depends strongly on the pH, the local amino acid sequence, and on the local structural environment of the amide8. Amides that are engaged in hydrogen bonding interactions (such as those present in α-helices and β-sheets) exchange more slowly than amides in unstructured regions of the protein that are exposed to bulk solvent. Thus, the extent of deuterium uptake is a reflection of the structure of the enzyme. Enzymes that are conformationally dynamic, or that undergo structural transitions upon ligand binding, would be expected to yield a measurable HDX response.
The mechanistic basis for the slow exchange rate of a structured amide is shown in Figure 25,8,9. In order to undergo HDX, the structured region must first transiently sample an unfolded conformation, such that the solvent molecules that catalyze HDX exchange via a specific acid/base chemical mechanism, have access to the exchangeable amide. Ultimately, the relative magnitudes of the chemical exchange rate (kchem) and the folding and refolding rates (kopen and kclose) determine the HDX rate measured in the experiment5,8. From this simple kinetic model, it is clear that extent of deuterium uptake will reflect the underlying conformational dynamics (as defined by kopen and kclose). Most HDX-MS experiments are performed in a bottom-up workflow where, following the exchange reaction, the protein of interest is digested into peptides and the deuterium uptake by each peptide is measured as an increase in mass7. In this way, HDX-MS allows perturbations to enzyme conformational dynamics to be mapped on the local spatial scale of peptides, allowing the researcher to assess how the perturbation alters dynamics in different regions of the enzyme of interest.
The advantages of the HDX-MS approach for elucidating protein structural dynamics are numerous. First, the method can be performed with small quantities of native protein or on protein complexes in systems with quaternary structure10. It is not even necessary for the enzyme preparation used in the assay to be highly purified11,12, as long as the bottom-up HDX-MS workflow provides a sufficient number of confidently identified peptides that cover the protein sequence of interest. Moreover, HDX-MS can provide information on conformational dynamics under near native conditions without the need for site-specific protein labeling as would be used in single molecule fluorescence studies13, and there is no size limit to the protein or protein complex that can be investigated (which makes approaches such as nuclear magnetic resonance [NMR] spectroscopy challenging)7,14. Finally, time-resolved HDX-MS methods can be employed to study intrinsically disordered proteins, which are difficult to study with X-ray crystallography15,16,17,18. The main limitation of HDX-MS is that the data is of low structural resolution. HDX-MS data are useful for pointing to where conformational dynamics are changing and for revealing coupled conformational changes, but they do not often provide much insight into the precise molecular mechanism driving the observed change. Recent advances in the combination of electron capture dissociation methods with protein HDX-MS data have shown promise for mapping exchange sites to single amino acid residues19, but follow up biochemical and structural studies are still often needed to provide clarity to structural models forwarded by HDX-MS data.
Below, a detailed protocol for the development of an HDX-MS assay is presented20. The sample preparation protocols presented below should be generally applicable to any protein that exhibits good solubility in aqueous buffers. More specialized sample preparation methods and HDX-MS workflows are available for proteins than need to be assayed in the presence of detergent or phospholipids21,22,23,24. Instrumental settings for HDX-MS data collection are described for a high-resolution quadrupole time-of-flight mass spectrometer coupled to liquid chromatography system. Data of similar complexity and resolution could be collected on any one of a number of commercially available liquid chromatography-mass spectrometry (LC-MS) systems. Key aspects of the data processing using a commercially available software package are also provided. We also present guidelines for data collection and analysis that are consistent with recommendations made by the broader HDX-MS community12. The described protocol is used to study the dynamic structural properties of HalM2, a lanthipeptide synthetase that catalyzes the multistep maturation of an antimicrobial peptide natural product20. We illustrate how HDX-MS can be used to reveal substrate binding sites and allosteric properties that have eluded previous characterization. Several other protocols on protein HDX-MS have been published in recent years25,26. Together with the present work, these earlier contributions should provide the reader some flexibility in experimental design.
Protocol
1. Preparation of deuterated reagents and enzyme stock solutions
- Prepare reagents needed for the HDX reactions (including any buffers, salts, substrates, ligands, etc.) as 100−200x concentrated stock solutions in D2O (99.9% atom fraction D). Prepare at least 50 mL of buffer stock solution.
NOTE: For characterization of HalM2, the following solutions were prepared: 500 mM MgCl2, 100 mM tris(2-carboxyethyl)phosphine (TCEP), 750 mM ATP (in HEPES buffer), 800 mM HEPES pD 7.1, 500 µM HalA2, and 500 mM AMPPNP. - Freeze and lyophilize the stock solutions to dryness.
- Re-dissolve in D2O, and repeat lyophilization cycle at least one additional time to replace as many of the exchangeable protons with deuterons as possible.
- Adjust the pD of the deuterated HEPES buffer stock to the desired value with concentrated NaOD/DCl, keeping in mind the following relationship27:
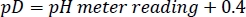
NOTE: The amide HDX rate is strongly dependent on the pL of the solution (pL = pH or pD)5. Different batches of buffer stock solutions need to be prepared, stored, and used in an identical manner to avoid slight pL drift between experiments. - Calculate the quantity of each reagent needed for a 300 µL HDX assay and store as single use aliquots at -80 °C.
- Prepare a concentrated enzyme stock solution (~100−200 µM) in protiated enzyme storage buffer using a centrifugal filter (Table of Materials) or equivalent device.
NOTE: The exact buffer and centrifugal filter molecular weight cutoff will depend on the protein/enzyme of interest. HalM2 is stored in 50 mM HEPES, pH 7.5, 100 mM KCl, and 10% glycerol. 10 kDa filters were used to prepare the concentrated enzyme. - Aliquot the enzyme into single use portions and store at -80 °C.
NOTE: This can be a stopping point. All stock solutions described in section 1 can be prepared in advance of the HDX reactions. If stored at -80 °C, most enzymes/deuterated stock solutions will be stable for many months.
2. Calibration of the HDX quench volume
- Prepare a 300 µL HDX reaction in D2O using the deuterated reagents and concentrated enzyme stocks prepared in section 1.
- Use a final enzyme concentration of 1−5 µM.
- Use a final deuterated HEPES buffer concentration of at least 50−100 mM.
- Ensure the concentrations of other components are sufficient to maintain the desired enzyme activity/function.
- Prepare 1 L of HDX quench solution (100 mM phosphate, 0.8 M guanidine-HCl, pH 1.9). Freeze and store in both 50 mL portions (for long term stock) and 1 mL portions (for single use aliquots).
NOTE: The exact composition of the quench buffer will depend on the enzyme that is used in the proteolysis step of the bottom-up HDX-MS workflow (step 3.3.3). The quench buffer given here is compatible with pepsin, the most commonly used protease for HDX-MS. If a different protease is used, check with the protease supplier to ensure buffer compatibility. - Calibrate the volume of quench buffer needed to adjust the final pL of the quenched HDX reaction mixture to a pH meter reading value of 2.3.
NOTE: The solvent H/D exchange rate of the peptide amide N-H bond is a pH-dependent process that is subject to both acid- and base-catalysis. The minimum exchange rate occurs at a value of pH 2.5 (pH meter reading = 2.3 for a 50:50 H2O:D2O mixture). Thus, a final pL value near 2.5 will minimize hydrogen back exchange that occurs during the bottom-up LC-MS analysis, thereby preserving the deuterium label in the peptides.- Mix 50 µL of the HDX reaction mixture from step 2.1 with 50 µL of quench buffer and measure the pL of the quenched mixture with a microtip electrode.
- Increase the volume of quench solution as needed to adjust the final pH meter reading to a value of 2.3.
- Once the appropriate quench volume has been determined, repeat the quenching process several times using fresh 50 µL aliquots from the HDX reaction (step 2.1) to ensure that a consistent final pL is achieved upon addition of a fixed quantity of quench buffer.
3. Preparation of reference samples and optimization of the bottom-up LC-MS workflow
- Prepare undeuterated reference samples for the protein of interest in triplicate in 0.5 mL tubes. Ensure that the final reaction mixture conditions are identical to those used in the authentic HDX reactions (step 2.1), except that the reactions are prepared in H2O using reagent stock solutions also prepared in H2O.
- Quench the samples as in step 2.3 by adding the appropriate volume of quench buffer to adjust the final pH to 2.5. Flash freeze the samples in liquid nitrogen and store at -80 °C until ready for analysis.
- Analyze the protiated enzyme reference samples using a bottom-up LC-MS workflow.
NOTE: Prior to executing these steps, the LC-MS system to be used for data acquisition should be properly calibrated and ready for use. The timing and temperature of all steps in the bottom-up LC-MS workflow must be rigorously controlled in order to minimize differences in back exchange between samples. With the MS instrumentation used in this protocol (Table of Materials and Supporting Information), most of the steps can be controlled through the instrument software. To ensure the collection of precise replicates, it is recommended to automate as many of the steps in the workflow as possible.- Remove an individual enzyme reference sample (prepared as in step 3.2) from the freezer and thaw at 37 °C for 1 min in a water bath.
- At precisely 2 min after removing the sample from the freezer and thawing, inject a 40 µL portion of the quenched reference sample into an ultra-performance liquid chromatography (UPLC) column (2.1 x 30 mm, 300 Å, 5 µM) containing a stationary phase functionalized with pepsin (an acid-stable protease).
- Digest the sample at a flow rate of 100 µL/min for 3 min at 15 °C using 0.1% formic acid in H2O (pH = 2.5) as the solvent.
- Collect the peptic peptides as they elute from the pepsin column onto a C18 trap column held at 0.4 °C to minimize back exchange.
- Pass the desalted peptic peptides from the trap column to a C18 analytical column (1 mm x 100 mm, 1.7 µM, 130 Å) held and operated at 0.4 °C for separation of the peptic peptides.
NOTE: Steps 3.3.3−3.3.5 can be automated by certain LC-MS systems used for HDX-MS data acquisition. Alternatively, these steps can be performed independently, keeping in mind that the timing and temperature of each step needs to be carefully controlled to achieve a consistently low back exchange. - Elute the C18 column with an acetonitrile/water/0.1% formic acid solvent system. Optimize the LC gradient for the protein of interest in order to maximize the separation of and to preserve the deuterium label in the peptic peptides.
NOTE: Gradient elution details are provided in the Supporting Information. - Subject the peptic digest to electrospray ionization (ESI) mass spectrometry.
NOTE: The source conditions provided in the Supporting Information will provide sufficient ionization for most peptic peptides.- Once ionized in the MS instrument, perform a gas phase ion mobility separation using nitrogen as the buffer gas to enhance the peak capacity of the method.
- Following the ion mobility separation, subject the peptic peptide precursor ions to an MSE workflow involving alternating cycles of low collision energy (4 V) and high collision energy (21−40 V).
NOTE: The alternating low and high collision energy regimes allow for the collection of MS data (low collision energy) simultaneously with MSMS data (high collision energy). This, in turn, allows for the time-correlation of precursor ions with their respective fragment ions. This correlation is essential for confident peptide identification described in section 4. - Detect the peptide precursor and fragment ions using a mass analyzer with a resolving power of at least 20,000.
- Simultaneous with the data acquisition, acquire MS data for a [Glu-1]-fibrinopeptide B (GluFib) external standard.
NOTE: The MS workflow described in step 3.3.7 is referred to as an MSE protocol. Complete instrumental settings for an MSE protocol that is suitable for bottom-up HDX-MS are provided in the Supporting Information.
- Assess the quality of the LC-MS data.
NOTE: Using the protocol described above and the instrumental settings provided in the Supporting Information, the reference samples should produce a total ion chromatogram with a maximum signal intensity of approximately 1 x 108. There should be many peptic peptides eluting between 3−9 min (Figure 3A−C). - Inject 40 µL of blank samples (0.1% formic acid in water) to clean the pepsin and analytical C18 columns.
NOTE: In general, 2−3 blanks should be sufficient. - Repeat steps 3.3.1−3.3.9 for each of the triplicate reference protein samples.
4. Processing the reference data and defining a peptide list
- Analyze the raw MSE data (step 3.3.7) using proteomics software (Table of Materials). Using the proteomics software, navigate to Libraries | Protein Sequence Databanks to define the protein database by importing the amino acid sequence of the protein of interest.
NOTE: The goal of this step is to search the reference MS data for peptic peptides derived from the protein of interest, and to use the MSMS data (acquired simultaneously with the MS data) to validate any putative peptide identifications. - Give a name to the protein sequence of interest. Import the protein sequence (in FASTA format). The software will perform an in silico digestion of the database protein to generate a list of peptides that will be used to search the LC-MS data.
- Define the processing parameters (located under the Library menu). Select Electrospray MSE as the data acquisition type. In the Lock Mass for Charge 2 field, enter 785.8426 for the m/z for the 2+ ion of [Glu-1]-fibrinopeptide B (GluFib) and click finish.
- Define the Workflow parameters (located under the Library menu).
- Select Electrospray MSE for the search type. Under the Workflow | Database Search Query heading, select the database protein created in step 4.2 in the Databank field.
- Change Primary Digest Reagent to nonspecific, and clear the Fixed Modifier Reagent field by holding the Ctrl button while clicking on Carbamidomethyl C.
- Specify the output directory by navigating to options | automation setup | Identity E. Check the boxes for Apex 3D and Peptide 3D Output and Ion Accounting Output and specify the desired directory.
- Process the reference sample data.
- On the left tool bar of the proteomics platform workspace, create a new plate by right clicking on Microtiter Plate. Highlight three wells in the microtiter plate (one for each reference sample collected in section 3). Left click in one well, hold and drag to three wells.
- Right click and select add raw data. In the window that appears, navigate to the directory containing the three reference files from section 3 and select them at the same time.
- Click on next and choose the processing parameters defined in step 4.3. Click on next and select the workflow parameters defined in step 4.4. Then click on finish.
- Once the raw data, processing parameters, and workflow parameters have been assigned to each well on the plate, the wells will appear blue. Select the wells, right click and select process latest raw data. Click on the right bottom corner of the window to track the processing of the data. Once the message No job to run appears, the processing is fully done.
- After data processing is complete, the wells in the plate will turn green. Right click on the wells and select view workflow results. A separate window will open for each reference data file.
- Inspect the data to ensure that the majority of MS signals in the reference sample data were successfully mapped to peptides predicted from the in-silico digestion of the protein of interest. Matched peptides will be colored blue in the output spectrum (Figure 4). Double-click on the OK filter and check that the percent coverage is greater than 99%.
NOTE: Upon processing, data output will be automatically saved with the file extension (raw_data_file_name_IA_final_peptide) in the directory specified in step 4.5. - Import the proteomics software output into the HDX-processing software (Table of Materials) for additional thresholding.
- Click on Data in the left corner of the HDX-processing software window. Click on import PLGS results and click on the add icon. Choose the processed data files from step 4.9 by navigating to the appropriate directory.
- Click on Next and specify the following parameters: minimum consecutive ions ≥ 2, mass error = 5 ppm, and file threshold = 3. Click on finish.
- Once satisfied with the thresholding parameters, save the HDX project. All HDX data will be imported into this project for analysis and display.
NOTE: The deuterium exchanged samples described in the next section will need to be processed with an identical LC-MS workflow. Therefore, before proceeding with HDX assays (section 5), ensure that the sample preparation (section 2), bottom-up LC-MS workflow (section 3) and data processing workflows (section 4) are providing the desired reproducibility and sequence coverage of the target protein. If any of these processes need to be changed to improve coverage, it is advised to return to step 2.1, prepare fresh reference samples in triplicate, and to repeat sections 2−4 (while making the necessary adjustments to the protocol) to ensure that each peptide can be reproducibly generated and detected.
5. Conducting HDX reactions
- Prepare workspace for HDX reactions.
- Pre-aliquot quench buffer into properly labeled 0.5 mL tubes. Prepare a different tube for every time point, every replicate, and every biochemical state to be analyzed. Use the appropriate volume of quench buffer from step 2.2 needed to adjust the final pH meter reading of a 50 µL portion of the HDX reaction to a value of 2.3.
- Briefly centrifuge the 0.5 mL tubs to transfer all of the quench buffer to the bottom of the tube. Place the tubes on ice.
- Fill a small Dewar with liquid nitrogen and keep adjacent to workspace.
- Prepare the HDX reactions. Ensure that there is sufficient reaction volume to collect the desired number of exchange time points (one 50 µL aliquot for each desired time point). Collect at least 4−5 time points over 3−4 orders of magnitude in time scale (e.g., quench times of 15 s, 60 s, 300 s [5 min], 1,800 s [30 min], and 14,400 s [4 h] provide adequate coverage of exchange dynamics for most enzymes).
- Pre-mix all of the deuterated components (minus enzyme) from step 1.1 in D2O.
- For each biochemical state to be examined (free enzyme, enzyme + ligand, enzyme + inhibitor, etc.), prepare HDX reactions in at least triplicate.
- Incubate the reaction mixtures in a temperature-controlled water bath at 25 °C for 10 min prior to addition of the enzyme.
NOTE: The enzyme should be prepared as a concentrated stock solution (~100−200 µM, step 1.6) so as to minimize the addition of protium into the HDX assay. - Upon adding the enzyme to a final concentration of 1−5 µM, start the timer. Carefully and quickly mix the solution using a 200 µL pipette to ensure that the enzyme is evenly distributed in the sample.
- At the desired exchange time points, remove 50 µL aliquots from the HDX reaction and mix quickly and evenly with the pre-aliquoted, ice cold quench buffer in a 0.5 mL tube.
NOTE: The mixing volumes and mixing procedure must be as precise and reproducible as possible to ensure that the desired final quench pL of 2.3 is achieved rapidly in all samples. Keeping the quench buffer ice cold will help to minimize back exchange upon denaturation of enzyme. - Immediately after quenching the HDX sample, cap the tube and flash freeze in liquid nitrogen.
- Continue collecting time points until all assays are complete, then transfer samples to the -80 °C freezer for storage.
NOTE: This can be a stopping point. After collecting all HDX time points, the samples can be stored at -80 °C until ready for LC-MS analysis. Ideally, triplicate HDX reactions should be performed for all biochemical states of interest on the same day. At a minimum, all replicate HDX reactions for a given biochemical state should be run in parallel on the same day.
- After collecting all of the quenched HDX time points, subject the samples to the optimized bottom-up LC-MS workflow developed as described in step 3.3. Inject HDX samples in a randomized order with an appropriate number of blanks between samples to ensure that any peptide carry over is minimal.
NOTE: HDX data do not need to be collected in MSE mode. Thus, the high collision energy segment (step 3.3.7.2) should be removed from the MS duty cycle. This should be the only alteration made to the workflow described in step 3.3. - Assess the quality of the HDX data as it is being collected.
- Ensure that chromatographic peaks present in the total ion chromatogram of the undeuterated reference samples appear at the same retention time in the deuterated samples (as in Figure 3D−F).
- Ensure that mass spectra summed over specific time intervals from reference and deuterated samples show evidence for deuteration (i.e., a shift in the isotopic envelope of individual peptides to higher m/z values in the deuterated samples [Figure 6]).
6. Processing HDX data
- Import the HDX data into the HDX project created in step 4.11 by clicking on Data | MS Files in the top tool bar.
- Click on New State and New Exposure as needed to define the biochemical states (e.g., free enzyme, enzyme + ligand, etc.) and deuterium exposure times, respectively, that are pertinent to the analysis.
- Click on New Raw to select the HDX data files to be analyzed. Assign the appropriate exchange times and biochemical state to each raw data file that is imported.
NOTE: The data can be imported and processed in batches, or all at once. Adding data to the project will not undo any analysis that has been previously performed within that project.
- Once the data files have been added, click finish to begin the data processing. After a short delay, the software will ask if the user wants to save the data before continuing. Click yes.
NOTE: The initial processing can take up to several hours depending on how many samples are being analyzed, how many peptides are in the final peptide list (step 4.10), the size of the chromatographic window, and the frequency of spectral acquisition. - If desired, alter the processing parameters in the Configuration menu to change the ion search parameters. Make sure to employ the same ion search parameters for all data in a given project.
7. Analysis and visualization of the HDX data
NOTE: Once the initial processing of the raw data has been completed (step 6.2), the HDX-processing software will have located peptides from the peptide list (generated in step 4.10) in each of the raw data files that were analyzed. Once the isotope distribution for a peptide in the list is located in a raw data file, the HDX-processing software represents each isotope with a “stick” (as in Figure 6C−E). The relative intensities of the sticks for a given peptide are then used to calculate the deuterium uptake relative to the reference spectra. While the HDX-processing software does an admirable job of properly assigning “sticks” to most peptides, significant manual curation of the deuterium uptake values will still be required.
- Analyzing peptide deuterium uptake values
- Select the first peptide in the peptide list and open the stacked spectral plot from the Views menu. Scroll up and down in the stacked spectra plot window to see the mass spectra for the selected peptide as function of deuterium exchange time (Figure 7D,E).
- Assign and unassign sticks as necessary using mouse clicks to ensure that the proper isotope distribution has been located in the data and that each isotope peaks has been assigned (assigned sticks will appear blue). Clicking on any of the spectra in the stacked spectral plot (Figure 7D,E) will allow the user to assign/unassign sticks in the active data viewer window (Figure 7C).
- Check the stick assignments for each charge state by toggling the charge state at the top of the stacked spectral plot window.
- Repeat steps 7.1.1−7.1.3 for each biochemical state of interest. The biochemical state can also be toggled at the top of the stacked spectral plot window. For the most accurate deuterium uptake difference measurements, ensure that sticks are assigned for the same set of charge states for each biochemical state.
- Repeat steps 7.1.1−7.1.4 for each peptide in the peptide list.
- Check the standard deviation of peptide deuterium uptake values using the coverage map.
- Access the coverage map from the Views menu, which displays each peptide in the peptide list mapped along the amino acid sequence of the protein of interest (Figure 8C). Color the peptides according to relative standard deviation (units of Da).
- Visually search the map for outlier peptides with high relative standard deviation. Click on the outlier peptides in the coverage map to populate the stacked spectral plots (Figure 8B) and data viewer window (Figure 8A) with the target peptide.
- Using the stacked spectral plot, carefully check that all charge states and all time points of the outlier peptide have appropriately assigned sticks.
NOTE: Most often, peptides with large standard deviations (>0.3 Da) have isotopic peaks that were not appropriately assigned sticks by the software (as indicated in Figure 8B). Assigning any missing sticks will generally enable the relative standard deviation of a peptide to be reduced to <0.3 Da. - Hide the peptide from the list if the relative standard deviation cannot be reduced to less than 0.3 Da.
- Export HDX difference data between two biochemical states for mapping onto a structural model of the protein of interest.
- Display the difference of interest in the coverage map. Right click on the coverage map to export the difference data to a .csv file. Export the state data (in .csv format) by navigating to Data | Export State Data in the main tool bar.
NOTE: Appropriate formatting of the difference data and state data files is provided in the Supporting Information. - Import the difference data, the state data, and the pdb file of the protein of interest into Deuteros28. Choose the 99% confidence interval, select enable sum, and process the data.
NOTE: MATLAB must be installed on the PC in order to run Deuteros. Deuteros will use the replicate measurements in the data set to calculate the standard deviation of the uptake data for each peptide. This standard deviation will be used to define the confidence interval for significant exchange, which will be displayed on the plots. - Under PyMOL Options, select export uptake | export to generate a Pymol script to map regions of significant exchange difference onto the pdb structure of the protein of interest using PyMOL software.
NOTE: Using the workflow described in this protocol, the 99% confidence interval for significant deuterium uptake difference for a given peptide at a single time point is typically 0.3−0.5 Da. The 99% confidence interval for the difference summed over all exchange time points is typically 0.7−1.0 Da.
- Display the difference of interest in the coverage map. Right click on the coverage map to export the difference data to a .csv file. Export the state data (in .csv format) by navigating to Data | Export State Data in the main tool bar.
Results
It is necessary to assess the quality of the proteolytic digestion and the reproducibility of the workflow for each set of sample injections. Thus, prior to performing HDX-MS assays, it is essential to establish effective conditions for the proteolysis of the protein of interest, for the separation of peptides using reverse phase liquid chromatography and gas phase ion mobility, and for the detection of peptides using MS. For this purpose, the reference samples for the protein of interest (collected in the absence of deu...
Discussion
The HDX-MS workflow presented in this protocol provides a remarkably robust platform for mapping the spatial distribution of structurally dynamic elements in proteins and for investigating how these dynamics change in response to perturbation (ligand binding, enzyme mutagenesis, etc.). HDX-MS holds several distinct advantages over other structural biology approaches that are commonly used to investigate conformational dynamics. Most notably, only small quantities of protein are needed. Using the workflow described herein...
Disclosures
We have nothing to disclose.
Acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council of Canada, the Fonds de Recherche du Quebec Nature et Technologie, the Canadian Foundation for Innovation, and McGill University start-up funds.
Materials
| Name | Company | Catalog Number | Comments |
| Reagents | |||
| [glu-1]-fibrinopeptide B (Glu-Fib) | BioBasic | NA | |
| 0.5 mL Amicon Ultracel 10k centrifugal filtration device (Millipore) | Milipore Sigma | UFC501096 | |
| acetonitrile | Fisher | A955-1 | |
| AMP-PNP | SIGMA | A2647-25MG | |
| ATP | SIGMA | a2383-5G | |
| D2O | ALDRICH | 435767-100G | |
| formic acid | Thermo Fisher | 28905 | |
| guanidine-HCl | VWR | 97063-764 | |
| HEPES | Fisher | BP310-1 | |
| Magnesium chloride | SiGMA-Aldrich | 63068-250G | |
| Potassium chloride | BioBasic | PB0440 | |
| potassium phosphate | BioBasic | PB0445 | |
| TCEP Hydrochloride | TRC Canada | T012500 | peptide was synthesized upon request |
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| software | |||
| Deuteros | Andy M C Lau, et al | version 1.08 | |
| DynamX | Waters | version 3.0 | |
| MassLynx | Waters | version 4.1 | |
| Protein Lynx Global Server (PLGS) | Waters | version 3.0.3 | |
| PyMOL | Schrödinger | version 2.2.2 | |
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| Instrument and equipment | |||
| ACQUITY UPLC BEH C18 analytical Column | Waters | 186002346 | |
| ACQUITY UPLC BEH C8 VanGuard Pre-column | Waters | 186003978 | |
| ACQUITY UPLC M-Class HDX System | Waters | ||
| HDX Manager | Waters | ||
| microtip pH electrode | Thermo Fisher | 13-620-291 | |
| Waters Enzymate BEH column or Pepsin solumn | Waters | 186007233 | |
| Waters Synapt G2-Si | Waters |
References
- Karplus, M., McCammon, J. A. Molecular dynamics simulations of biomolecules. Nature Structural & Molecular Biology. 9 (9), 646-652 (2002).
- Campbell, E., et al. The role of protein dynamics in the evolution of new enzyme function. Nature Chemical Biology. 12 (11), 944-950 (2016).
- Tokuriki, N., Tawfik, D. S. Protein dynamism and evolvability. Science. 324 (5924), 203-207 (2009).
- Wales, T. E., Engen, J. R. Hydrogen exchange mass spectrometry for the analysis of protein dynamics. Mass Spectrometry Reviews. 25 (1), 158-170 (2006).
- Konermann, L., Pan, J., Liu, Y. H. Hydrogen exchange mass spectrometry for studying protein structure and dynamics. Chemical Society Reviews. 40 (3), 1224 (2011).
- Katta, V., Chait, B. T. Conformational changes in proteins probed by hydrogen-exchange electrospray-ionization mass spectrometry. Rapid Communications in Mass Spectrometry. 5 (4), 214 (1991).
- Zhang, Z., Smith, D. L. Determination of amide hydrogen exchange by mass spectrometry: a new tool for protein structure elucidation. Protein Science. 2 (4), 522 (1993).
- Smith, D. L., Deng, Y., Zhang, Z. Probing the non-covalent structure of proteins by amide hydrogen exchange and mass spectrometry. Journal of Mass Spectrometry. 32 (2), 135-146 (1997).
- Bai, Y., Milne, J. S., Mayne, L., Englander, S. W. Primary structure effects on peptide group hydrogen exchange. Proteins. 17 (1), 75-86 (1993).
- Xiao, K., et al. Revealing the architecture of protein complexes by an orthogonal approach combining HDXMS, CXMS, and disulfide trapping. Nature Protocols. 13 (6), 1403-1428 (2018).
- Smith, D. L., Deng, Y., Zhang, Z. Probing the non-covalent structure of proteins by amide hydrogen exchange and mass spectrometry. Journal of Mass Spectrometry. 32 (2), 135-146 (1997).
- Masson, G. R., et al. Recommendations for performing, interpreting and reporting hydrogen deuterium exchange mass spectrometry (HDX-MS) experiments. Nature Methods. 16 (7), 595-602 (2019).
- Liu, J., et al. An Efficient Site-Specific Method for Irreversible Covalent Labeling of Proteins with a Fluorophore. Scientific Reports. 5, 16883 (2015).
- Marion, D., et al. Overcoming the overlap problem in the assignment of proton NMR spectra of larger proteins by use of three-dimensional heteronuclear proton-nitrogen-15 Hartmann-Hahn-multiple quantum coherence and nuclear Overhauser-multiple quantum coherence spectroscopy: application to interleukin 1.beta. Biochemistry. 28 (15), 6150-6156 (1989).
- Balasubramaniam, D., Komives, E. A. Hydrogen-exchange mass spectrometry for the study of intrinsic disorder in proteins. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1834 (6), 1202-1209 (2013).
- Goswami, D., et al. Time window expansion for HDX analysis of an intrinsically disordered protein. Journal of the American Society for Mass Spectrometry. 24 (10), 1584-1592 (2013).
- Keppel, T. R., Howard, B. A., Weis, D. D. Mapping Unstructured Regions and Synergistic Folding in Intrinsically Disordered Proteins with Amide H/D Exchange Mass Spectrometry. Biochemistry. 50 (40), 8722-8732 (2011).
- Mitchell, J. L., et al. Functional Characterization and Conformational Analysis of the Herpesvirus saimiri Tip-C484 Protein. Journal of Molecular Biology. 366 (4), 1282-1293 (2007).
- Pan, J., Han, J., Borchers, C. H., Konermann, L. Hydrogen/deuterium exchange mass spectrometry with top-down electron capture dissociation for characterizing structural transitions of a 17 kDa protein. Journal of the American Chemical Society. 131 (35), 12801-12808 (2009).
- Habibi, Y., Uggowitzer, K. A., Issak, H., Thibodeaux, C. J. Insights into the Dynamic Structural Properties of a Lanthipeptide Synthetase using Hydrogen-Deuterium Exchange Mass Spectrometry. Journal of the American Chemical Society. 141 (37), 14661-14672 (2019).
- Hebling, C. M., et al. Conformational analysis of membrane proteins in phospholipid bilayer nanodiscs by hydrogen exchange mass spectrometry. Analytical Chemistry. 82 (13), 5415-5419 (2010).
- Duc, N. M., et al. Effective application of bicelles for conformational analysis of G protein-coupled receptors by hydrogen/deuterium exchange mass spectrometry. Journal of the American Society for Mass Spectrometry. 26 (5), 808-817 (2015).
- Canul-Tec, J. C., et al. Structure and allosteric inhibition of excitatory amino acid transporter 1. Nature. 544 (7651), 446-451 (2017).
- Reading, E., et al. Interrogating Membrane Protein Conformational Dynamics within Native Lipid Compositions. Angewandte Chemie International Edition. 56 (49), 15654-15657 (2017).
- Hentze, N., Mayer, M. P. Analyzing protein dynamics using hydrogen exchange mass spectrometry. Journal of Visualized Experiments. (81), e50839 (2013).
- Lento, C., et al. Time-resolved ElectroSpray Ionization Hydrogen-deuterium Exchange Mass Spectrometry for Studying Protein Structure and Dynamics. Journal of Visualized Experiments. (122), e55464 (2017).
- Schowen, K. B., Schowen, R. L. Solvent isotope effects on enzyme systems. Methods in Enzymology. 87, 551-606 (1982).
- Lau, A. M. C., Ahdash, Z., Martens, C., Politis, A. Deuteros: software for rapid analysis and visualization of data from differential hydrogen deuterium exchange-mass spectrometry. Bioinformatics. 35 (7), 3171-3173 (2019).
- Houde, D., Berkowitz, S. A., Engen, J. R. The utility of hydrogen/deuterium exchange mass spectrometry in biopharmaceutical comparability studies. Journal of Pharmaceutical Sciences. 100 (6), 2071 (2011).
- Burkitt, W., O'Connor, G. Assessment of the repeatability and reproducibility of hydrogen/deuterium exchange mass spectrometry measurements. Rapid Communications in Mass Spectrometry. 22 (23), 3893-3901 (2008).
- Rob, T., Gill, P. K., Golemi-Kotra, D., Wilson, D. J. An electrospray ms-coupled microfluidic device for sub-second hydrogen/deuterium exchange pulse-labelling reveals allosteric effects in enzyme inhibition. Lab on a Chip. 13, 2528-2532 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved