A subscription to JoVE is required to view this content. Sign in or start your free trial.
Single-Cell Optical Action Potential Measurement in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes
In This Article
Summary
Here we describe optical acquisition and characterization of action potentials from induced pluripotent stem cell derived cardiomyocytes using a high-speed modular photometry system.
Abstract
Conventional intracellular microelectrode techniques to quantify cardiomyocyte electrophysiology are extremely complex, labor intensive, and typically carried out in low throughput. Rapid and ongoing expansion of induced pluripotent stem cell (iPSC) technology presents a new standard in cardiovascular research and alternate methods are now necessary to increase throughput of electrophysiological data at a single cell level. VF2.1Cl is a recently derived voltage sensitive dye which provides a rapid single channel, high magnitude response to fluctuations in membrane potential. It possesses kinetics superior to those of other existing voltage indicators and makes available functional data equivalent to that of traditional microelectrode techniques. Here, we demonstrate simplified, non-invasive action potential characterization in externally paced human iPSC derived cardiomyocytes using a modular and highly affordable photometry system.
Introduction
Electrophysiological modeling of cardiomyocytes and the construction of efficient platforms for cardiac drug screening is essential for the development of therapeutic strategies for a variety of arrhythmic disorders. Rapid expansion of induced pluripotent stem cell (iPSC) technology has produced promising inroads into human disease modelling and pharmacological investigation using isolated patient derived cardiomyocytes (iPSC-CM). “Gold standard” techniques for electrophysiological characterization of these cells through patch-clamp (current-clamp) can quantify action potential (AP) morphology and duration, however, this method is incredibly complex and sl....
Protocol
1. Cellular preparations
NOTE: Human iPSCs used in this protocol were derived from healthy donors and differentiated in monolayers using fully defined small molecule modulation of WNT signaling and lactate purification techniques as previously described12,13,14. iPSC-CMs were maintained every 2-3 days with a culture medium outlined below.
- Prepare a culture medium of basal medium (RPMI 1640.......
Representative Results
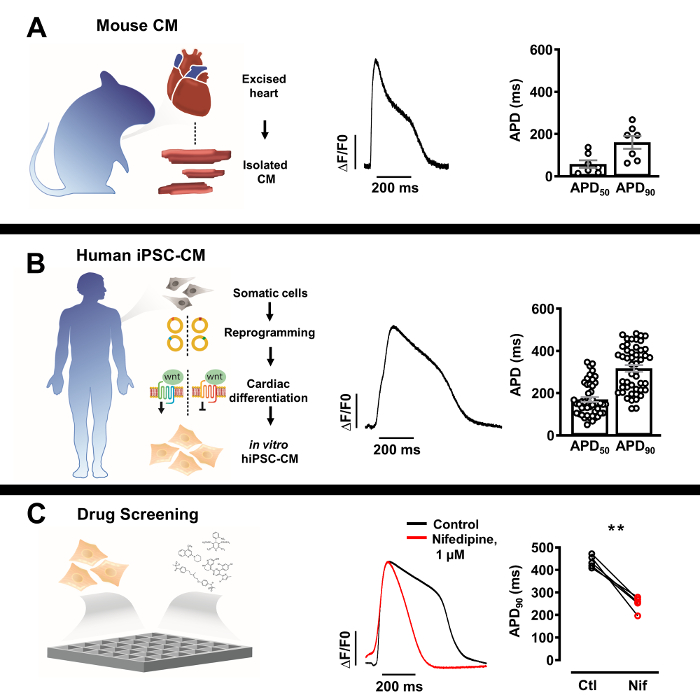
Figure 3: Optical action potential (AP) profiles of isolated native cardiomyocytes and human induced pluripotent stem cell derived cardiomyocytes (iPSC-CM). (A) Representative optical AP of a single murine cardiomyocyte (center) with Mean ± SEM of APD50 and APD90 (n = 7, right). (B) Representative optical AP of a singl.......
Discussion
Here we describe a basic protocol to easily acquire detailed AP profiles from isolated iPSC-CMs suitable for electrophysiological modelling and cardiac drug screening. We detect regular, robust APs from our sparsely seeded iPSC-CMs which suggests both indicator functionality and methodological fidelity.
Due to the wide spectrum of commercial methodologies for iPSC reprogramming and lack of standardization for cardiac differentiation protocols, iPSC based models can show immense variability in .......
Acknowledgements
The authors would like to acknowledge Cairn Research Ltd. for their kind financial contribution which covered production costs of this publication. In addition, we thank Ms. Ines Mueller and Ms. Stefanie Kestel for their excellent technical support.
The authors’ research is supported by the German Center for Cardiovascular Research (DZHK), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, VO 1568/3-1, IRTG1816 RP12, SFB1002 TPA13 and under Germany’s Excellence Strategy - EXC 2067/1- 390729940) and the Else-Kröner-Fresenius Stiftung (EKFS 2016_A20).
....Materials
| Name | Company | Catalog Number | Comments |
| Reagents | |||
| 0.25 Trypsin EDTA | Gibco | 25200056 | |
| B27 Supplement | Gibco | 17504044 | |
| CaCl2 | Carl Roth | HN04.2 | |
| D(+)-Glucose anhydrous BioChemica | ITW Reagents | A1422 | |
| Fetal Bovine Serum | Gibco | 10270-106 | |
| FluoVolt Membrane Potential Kit | Invitrogen | F10488 | |
| HEPES | Carl Roth | HN77.4 | |
| KCl | Sigma-Aldrich | 6781.1 | |
| Lamanin | Sigma-Aldrich | 114956-81-9 | |
| Matrigel | BD | 354230 | |
| NaCl | Sigma-Aldrich | 9265.2 | |
| Nifedipine | Sigma-Aldrich | 21829-25-4 | |
| Penicillin/Streptomycin | Invitrogen | 15140 | |
| ROCK Inhibitor Y27632 | Stemolecule | 04-0012-10 | |
| RPMI 1640 Medium | Gibco | 61870010 | |
| Versene EDTA | Gibco | 15040033 | |
| Equipment | |||
| 495LP Dichroic Beamsplitter | Chroma Technology | ||
| Axopatch 200B Amplifier | Molecular Devices | ||
| Circle Coverslips, Thickness 0 | Thermo Scientific | CB00100RA020MNT0 | |
| Digidata 1550B | Molecular Devices | ||
| Dual OptoLED Power Supply | Cairn Research | ||
| ET470/40x Excitation Filter | Chroma Technology | ||
| ET535/50m | Chroma Technology | ||
| Etched Neubauer Hemacytometer | Hausser Scientific | ||
| Filter Cubes | Cairn Research | ||
| IX73 Inverted Microscope | Olympus | ||
| MonoLED | Cairn Research | ||
| Multiport Adaptors | Cairn Research | ||
| Myopacer Cell Stimulator | IonOptix | ||
| Optomask Shutter | Cairn Research | ||
| Optoscan System Controller | Cairn Research | ||
| PH-1 Temperature Controlled Platform | Warner Instruments | ||
| Photomultiplier Detector | Cairn Research | ||
| PMT Amplifier Insert | Cairn Research | ||
| PMT Supply Insert | Cairn Research | ||
| RC-26G Open Bath Chamber | Warner Instruments | ||
| SA-OLY/2AL Stage Adaptor | Olympus | ||
| T565lpxr Dichroic Beamsplitter | Chroma Technology | ||
| T660lpxr Dichroic Beamsplitter | Chroma Technology | ||
| TC-20 Dual Channel Temperature Controller | npi Electronic | ||
| UPLFLN 40X Objective | Olympus | ||
| USB 3.0 Colour Camera | Imaging Source | ||
| Software | |||
| Clampex 11.1 | Molecular Devices | ||
| Clampfit 11.1 | Molecular Devices | ||
| IC Capture 2.4 | Imaging Source | ||
| Prism 8 | Graphpad |
References
- Miller, E. W. Small molecule fluorescent voltage indicators for studying membrane potential. Current Opinion in Chemical Biology. 33, 74-80 (2016).
- Liang, P., et al.
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved