A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Nasal Brushing Sampling and Processing Using Digital High Speed Ciliary Videomicroscopy – Adaptation for the COVID-19 Pandemic
In This Article
Summary
To guarantee a successful and high-quality ciliary functional analysis for PCD diagnosis, a precise and careful method for respiratory epithelium sampling and processing is essential. To continue providing PCD diagnostic service during the COVID-19 pandemic, the ciliary videomicroscopy protocol has been updated to include appropriate infection control measures.
Abstract
Primary Ciliary Dyskinesia (PCD) is a genetic motile ciliopathy, leading to significant otosinopulmonary disease. PCD diagnosis is often missed or delayed due to challenges with different diagnostic modalities. Ciliary videomicroscopy, using Digital High-Speed Videomicroscopy (DHSV), one of the diagnostic tools for PCD, is considered the optimal method to perform ciliary functional analysis (CFA), comprising of ciliary beat frequency (CBF) and beat pattern (CBP) analysis. However, DHSV lacks standardized, published operating procedure for processing and analyzing samples. It also uses living respiratory epithelium, a significant infection control issue during the COVID-19 pandemic. To continue providing a diagnostic service during this health crisis, the ciliary videomicroscopy protocol has been adapted to include adequate infection control measures.
Here, we describe a revised protocol for sampling and laboratory processing of ciliated respiratory samples, highlighting adaptations made to comply with COVID-19 infection control measures. Representative results of CFA from nasal brushing samples obtained from 16 healthy subjects, processed and analyzed according to this protocol, are described. We also illustrate the importance of obtaining and processing optimal quality epithelial ciliated strips, as samples not meeting quality selection criteria do now allow for CFA, potentially decreasing the diagnostic reliability and the efficiency of this technique.
Introduction
Primary ciliary dyskinesia (PCD) is an inherited heterogeneous motile ciliopathy, in which respiratory cilia are stationary, slow or dyskinetic, leading to impaired mucociliary clearance and chronic oto-sino-pulmonary disease1,2,3,4. The clinical manifestations of PCD are chronic wet cough and chronic nasal congestion starting in early infancy, recurrent or chronic upper and lower respiratory tract infections leading to bronchiectasis, and recurrent or chronic otitis media and sinusitis5,6,7. Approximately half of PCD patients present with organ laterality defects such as situs inversus or situs ambiguus. Some patients also present with infertility issues due to immotile sperm in men and immotile cilia in the Fallopian tubes in women1,2,8. PCD is rare, but the prevalence is difficult to define, and ranges from 1:10,000 to 1:20,0009,10. However, the real prevalence of PCD is thought to be higher due to difficulties in diagnosis and a lack of clinical suspicion. Symptoms of PCD mimic common respiratory manifestations of other acute or chronic respiratory conditions, and the diagnostic challenges of confirming the diagnosis are well known, leading to inadequate treatment and follow-up2,5,9,11.
Ciliary videomicroscopy, using Digital High-Speed Videomicroscopy (DHSV), is one of the diagnostic tools for PCD4,8,12,13. DHSV is considered the optimal method to perform ciliary functional analysis (CFA), comprising of ciliary beat frequency (CBF) and beat pattern (CBP) analysis2,14,15,16. DHSV uses living respiratory epithelium, usually obtained from nasal brushing13.
In view of the current COVID-19 outbreak, confirmation of a PCD diagnosis is now even more important as evidence suggests that underlying respiratory disease may lead to worse outcomes following COVID-19 infection17,18. A safe and efficient PCD diagnostic service during the current pandemic will also allow confirmed PCD patients to benefit from additional protective measures, compared with the general population19.
Transmission of COVID-19 occurs primarily through droplet spread20. High potential of transmission from asymptomatic (or minimally symptomatic) patients is suggested by the high viral load in nose sample20. Additionally, if viral particles become aerosolized, they stay in the air for at least 3 hours21. Therefore, respiratory healthcare workers are exposed to a high reservoir of viral load while performing clinical care and sample collection for diagnostic techniques22. Furthermore, manipulation of living respiratory samples exposes the technician to COVID-19 contamination. While best-practice recommendations for respiratory physicians and ENT surgeons caring for COVID-19 patients are being implemented23, there is a lack of recommendations for performing DHSV during the COVID-19 pandemic.
In order to continue providing a PCD diagnostic service, while ensuring the safety of the healthcare worker (performing sample collection) and technician (performing sample processing), the ciliary videomicroscopy protocol had to be adapted during the COVID-19 pandemic. The technique of ciliary videomicroscopy is currently limited to research service and specialized diagnostic centers, as CFA requires extensive training and experience. Furthermore, currently, there is a lack of standardization and precise operating procedure for processing and analyzing samples using DHSV4,13.
The aim of this paper is to describe standard operating procedures for DHSV, with particular reference to infection control measures and safety when sampling and processing living nasal epithelium. This will allow for high-quality PCD diagnosis and care to continue, despite the current COVID-19 outbreak.
Protocol
Approval was obtained from the Liege hospital-faculty ethics committee and the University Department for Hygiene and Health Protection at Work.
1. Sampling respiratory ciliated epithelium
- Ensure that subjects are free of infection for at least 4-6 weeks, and free of nasal and inhaled medication, before sampling.
- Prepare supplemented M199 preparation: Supplement Cell Culture Medium 199 (M199) (500 mL) with antibiotic solution (5 mL of streptomycin/penicillin (50 μg/mL)) and antifungal solution (5 mL of amphotericin B (2.5μg/mL)).
- Prepare 2 (one for each nostril) 15 mL conical tubes with lids, and fill each of them with 3 mL of supplemented M199.
- Prepare a bronchial cytology brush (thickness: 2 mm and length: 11 mm). Cut the end of the wire to ensure that the brush is about 15 cm long (Figure 1A,B). To hold the brush when performing the nasal brushing, use a Weil-Blakesley nasal forceps(Figure 1B).
- COVID-19 adaptation: Avoid processing a living nasal epithelium sample of unknown status for COVID-19, test the patient for COVID-19 48 to 72 hours before the nasal brushing for ciliary videomicroscopy. This COVID-19 test consists of polymerase chain reaction from a nasopharyngeal swab sample24,25. As the patient’s status for COVID-19 is unknown at this point, physician and staff members must be adequately protected23,26, including FFP2 mask, gloves, face shield or goggles, and long-sleeved water-resistant gown. In case of unavailable, impossible or doubtful PCR testing, made all processing of nasal brushing in L2 bio-safety laboratory. In case of positive COVID-19 status, postpone PCD diagnosis testing and consider alternative approaches to manage the patient.
CAUTION: This nasopharyngeal swab sampling for COVID-19 testing might induce secondary ciliary dyskinesia by damaging nasal respiratory ciliary epithelium27,28. To avoid this, introduce a thin cotton swab into the nasal cavity up to the nasopharynx under rigid endoscopic control, avoiding hurting the turbinates or the septum. The sample is then taken from the nasopharynx and remove the cotton swab under the control of the rigid endoscope. With adequate equipment, a 0° rigid endoscopy is easily performed in adults and children without trauma.
2. Obtaining respiratory ciliated epithelium specimens
COVID-19 adaptation: Even if the COVID-19 status of the patient is negative, due to false-negative rate, the patient is asked to keep a surgical mask on his/her mouth during the procedure, and gloves, FFP2 mask and face shield are worn by the physician.
- Nasal brushing preparation
- Ask the patient to blow his/her nose.
- Perform nasal brushing under nasal endoscopy or blinded. If using a nasal endoscopy, examine the 2 nostrils prior to the nasal brushing (do not repeat if done 48-72 previously for COVID-19 nasal swab). Examination makes it possible to verify the condition of mucosa (a high degree of inflammation might cause bleeding when nasal brushing is performed, …), the condition of inferior turbinate (to exclude the presence of telangiectasia for example), and if the septum nasal is straight (Figure 1C).
- Ask the patient to lie down, or to sit comfortably, the head resting backwards on the chair (because the nasal brushing causes a reflex to move the head back). A second carer hold the head during the nasal brushing, particularly in children.
- Shake the brush in the supplemented M199 prior to nasal brushing (moistening the brush reduces irritation from brushing).
NOTE: The brush might be moistened within the supplemented M199; if the patient is allergic to antibiotics (penicillin and streptomycin are present in the supplemented cell culture medium), moisten the brush in saline.
- Nasal brushing
- Gently insert the nasal brushing without local or general anesthesia13. If using nasal endoscopy, place the endoscope at the entrance of nose to visualize the inferior nasal turbinate, then insert the cytology brush in the nose. If performing a “blinded” nasal brushing, insert the brush into the nose, following the nasal floor (Figure 1D).
NOTE: Some diagnostic centers use local anesthesia with a tampon of naphazoline to perform nasal brushing. - Move the brush posteriorly and anteriorly several times over the posterior part of the inferior nasal turbinate and then withdraw. The operator should feel that the brush rubs the epithelium, and the patient might feel unilateral watery eye on the side of the brushing.
NOTE: If the nasal brushing is performed too anteriorly, no ciliated cells will be obtained, as the anterior nasal cavity is lined with a transitional non-ciliated epithelium. - After sampling, immediately place nasal brushing specimens within the culture medium. Respiratory epithelial strips obtained are dislodged by agitating the brush in the tube containing the supplemented M199, then close the tube (Figure 1E).
- COVID-19 adaptation: Do not dislodge epithelial strips by agitating the brush in the supplemented M199 immediately after sampling. Place the brush in the tube, cut the wire so that it can fit completely inside the tube, and close the tube immediately. Place the sample in an airtight double bag.
- Gently insert the nasal brushing without local or general anesthesia13. If using nasal endoscopy, place the endoscope at the entrance of nose to visualize the inferior nasal turbinate, then insert the cytology brush in the nose. If performing a “blinded” nasal brushing, insert the brush into the nose, following the nasal floor (Figure 1D).
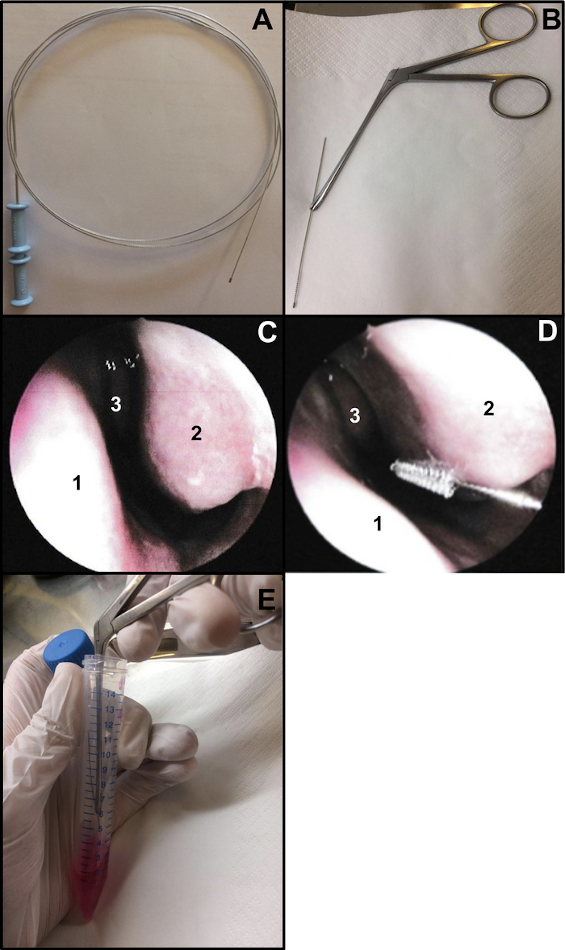
Figure 1: Nasal brushing technique. (A) Entire bronchial cytology brush (B) Ready-to- brush: the brushing end of the wire is cut (about 15 cm long) and held by a Weil-Blakesley nasal forceps(C) Endoscopic view of the nasal cavity: septum (1) inferior turbinate (2) and middle turbinate (3) (D) Nasal brushing is performed on the posterior part of the inferior turbinate (2). Nasal septum (1) Middle turbinate (3). (E) The respiratory epithelial strips are dislodged by shaking the brush in the supplemented M199 cell culture medium. Please click here to view a larger version of this figure.
3. Respiratory ciliated epithelium processing
- Analyze nasal brushing samples under microscope within 9 hours post-sampling, as both CBF and CBP are stable within this time frame (unpublished data).
- Use an upright or an inverted light microscope, with a x100 oil-immersion phase-contrast or an interference contrast lens. Ideally, place the microscope on an anti-vibration table because ciliary beating may be subject to artifacts due to external vibrations (e.g. from the laboratory bench)13.
COVID-19 adaptation: The operator uses personal protective equipment to perform nasal processing, including FFP2 mask, gloves, and long-sleeved water-resistant gown.
- Prepare the visualization chamber.
- Suspend the ciliated epithelial strips in a lab-built open visualization chamber, allowing cilia to beat freely while being analyzed under the microscope. This chamber is created by the separation of a cover slip (22 mm x 40 mm) and a glass slide by two adjacent square cover slips (20 mm x 20 mm), separated by a distance of 15 mm, and glued on the glass slide12 (Figure 2, Figure 4A).
COVID-19 adaptation: The lab-built chamber described above is open, and allows gas and humidity exchange between the sample and the environment13. In the context of the COVID-19 pandemic, it is possible to use a closed visualization chamber using a double-sided stuck spacer, 0.25 mm depth (Figure 3, Figure 4B). The spacer is stuck on the glass slide, and then a cover slip (22 mm x 40 mm) is stuck on top of the spacer.
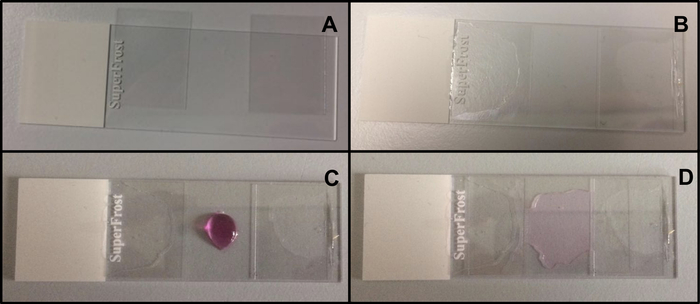
Figure 2: Mounting of the lab-built open chamber. (A) The 2 square coverslips (20 mm x 20 mm) are placed on the glass slide. (B) The square cover slips are separated by a distance of about 15 mm, and glued on the glass slide. (C) The chamber is filled between the two adjacent square cover slips with a small sample (approximately 60 μL) of ciliated epithelium in supplemented M199. (D) A long rectangular coverslip (22 mm x 40 mm) is placed on the two adjacent square cover slips, and covers the chamber. Please click here to view a larger version of this figure.
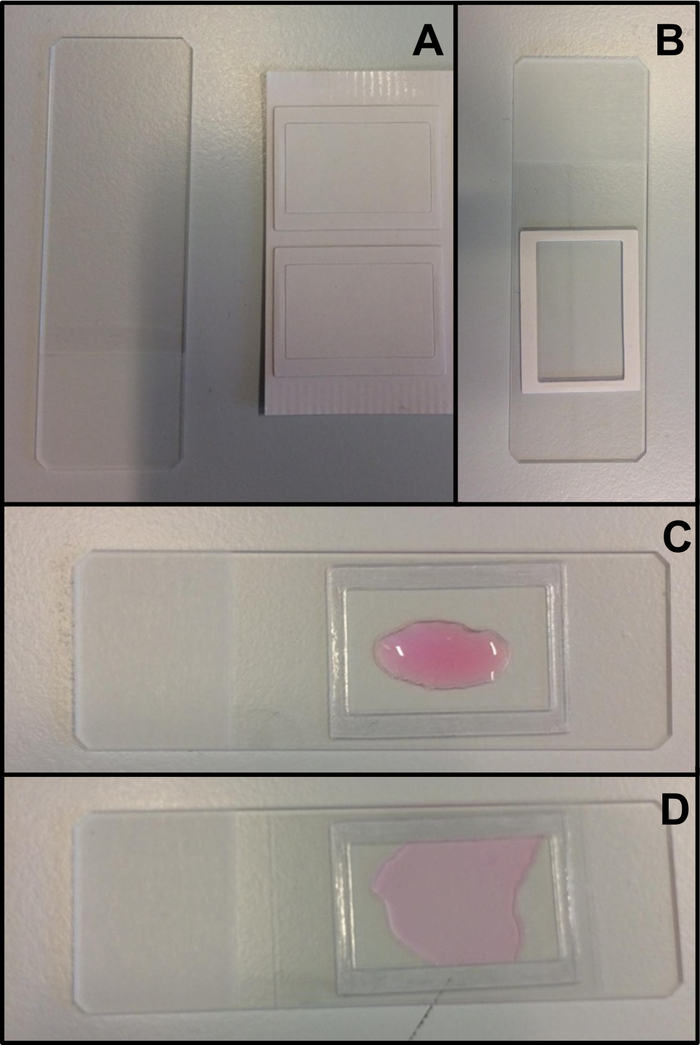
Figure 3: Mounting of the closed chamber using a double-sided stuck spacer. (A) The glass slide and the double-side stuck spacer. (B) The protection is removed on one side of the spacer, and the spacer is then stuck on the glass slide. (C) The protection is removed from the other side of the double-sided stuck spacer, and then the spacer is filled with a small sample (approximately 60 μL) of ciliated epithelium in supplemented M199. (D) A long rectangular coverslip (22 mm x 40 mm) is stuck on the spacer, and closes the chamber. Please click here to view a larger version of this figure.
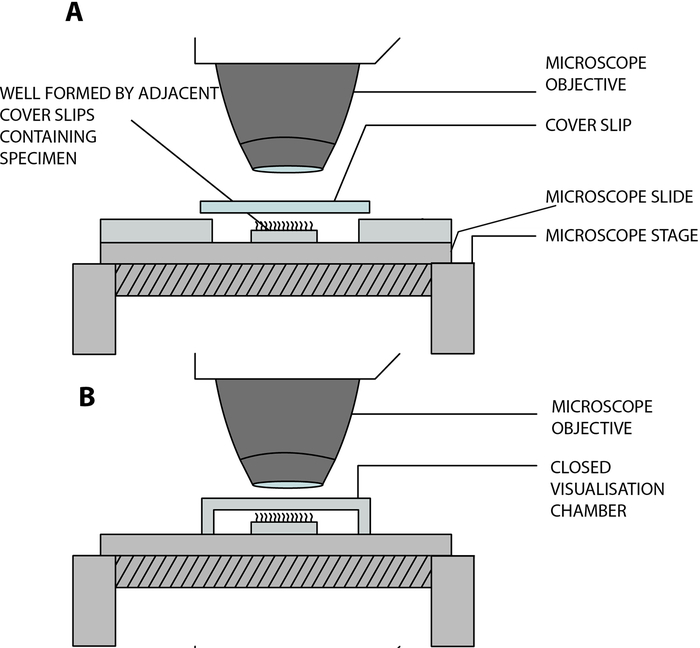
Figure 4: Schematic diagram showing the main visualization chambers used to perform ciliary videomicroscopy using digital high-speed videomicroscopy (DHSV). (A) The open hanging drop technique: the ciliated sample is suspended in a drop of cell culture medium in an open chamber created by the separation of a coverslip and a glass slide by two adjacent coverslips. (B) The closed hanging drop technique: the ciliated sample is suspended in a drop of cell culture medium in a closed chamber created by a spacer sandwiched between a glass side and a cover slip. The spacer sticks firmly on both the glass slide and the cover slip. Reproduced and modified from Kempeneers et al.13. Please click here to view a larger version of this figure.
- Control of temperature
- Surround the microscope with bubble wrap (Figure 5A,B).
- Attach the lens heater around the objective using a Velcro strap (Figure 5C)
- Turn on the lens heater controller 1 hour before performing the control temperature check.
- Turn on the microscope and check that the microscope set up is done, as the amount of the light through the sample can change the temperature on the slide.
- Turn on the heated box controller (Figure 5D).
- Check that the reference probe functions properly before starting. Hold the reference probe tip between fingers; it should measure the body temperature.
- Put free media into the middle of the slide, between the two adjacent square cover slips (20 mm x 20 mm) glued on it.
- Place the reference probe tip in the supplemented M199. Cover with a rectangular coverslip (22 mm x 40 mm). Be sure that the probe is completely surrounded by media (otherwise the temperature could drop).
- COVID-19 adaptation: To perform the temperature control in the closed chamber using a spacer, cut one side of the spacer (this hole must be the same size as the reference probe). Stick the spacer onto the glass slide, place free media in the middle of the spacer. Place the tip of the reference probe into the solution, through the hole of the spacer, then stick a rectangular coverslip (22 mm x 40 mm) on the spacer.
- Place the slide in the plate of the heated box. Close the heated box with the lid.
- Add oil on the oil-immersion objective.
- Place the heated box on the microscope stage.
- Adjust the temperature of the plate and the lid (the temperature of the lid should be 2 °C higher than the temperature of the plate to avoid condensation) to measure 37 °C with the reference probe within the medium.
- Wait 5 minutes (time required to raise the temperature of the sample to 37 °C).
- Adjust the objective, moving it closer to the slide until touching the coverslip with the tip of the lens.
- Move the objective in order to see the middle of the probe in the microscope.
NOTE: Be sure that the probe is seen on the computer screen (in order to check that the camera system works before looking at the ciliated sample). When viewing the middle of the probe, the screen is completely black. - Adjust the temperature of the lens heater (to compensate for the loss of temperature when the oil-immersion lens is in contact with the coverslip). Be sure to measure 37 °C with the reference probe within the medium when the objective touches the cover slip.
NOTE: Ideally, work in a room with a controlled temperature, so that these temperatures set up do not change. If the temperature of the room is not controlled, you should perform this temperature control check every day before performing ciliary videomicroscopy. - After checking the temperature, remove the slide from the heated box.
- Clean the slide and the tip of reference probe with alcohol and put away.
- Clean the lens with isopropanol and lens cleaning tissues with circular motions.

Figure 5: Equipment used in the DHSV laboratory. (A) The microscope equipped with a 100x oil-immersion phase-contrast lens, is placed on an anti-vibration table to avoid that external vibrations cause artifacts for ciliary functional analysis (B) The microscope is surrounded by bubble wrap to prevent heat loss from ambient air. (C) The oil immersion objective creates heat loss. this can be prevented using a lens heater (arrows). (D) The sample is heated using a heating box. Please click here to view a larger version of this figure.
4. Preparation of the respiratory ciliated epithelial samples
- Shake the tube gently to allow cilia to spread out throughout the tube (to avoid cilia to be stuck on other ciliated strips, mucus or debris, which prevent them from beating freely).
NOTE: This step is essential to obtain “optimal edges” of ciliated epithelium (Figure 12). - Withdraw approximately 50 μL of ciliated epithelium in supplemented M199 at the middle of the tube with a pipette.
- Put the sample on the lab-built chamber (between the two adjacent square cover slips (20 mm x 20 mm)) and cover with a rectangular coverslip (22 mm x 40 mm). Be careful not to add bubbles.
- COVID-19 adaptation: Carry out steps 4.1-4.3 in a microbiological safety cabinet. Procedure in the microbiological safety cabinet.
- Switch on the microbiological safety cabinet 10 minutes before preparing the sample (to make sure that the environment is sterile).
- Before any handling, disinfect the entire microbiological safety cabinet with 70% ethanol.
- Disinfect all necessary material with 70% ethanol before placing in the microbiological safety cabinet.
- Open the 15 mL conical tubes containing the samples only once under the microbiological safety cabinet, then dislodge epithelial strips by agitating the brush (using Weil-Blakesley nasal forceps) in supplemented M199.
- Stick the spacer on the glass slide and remove the protection from the double-sided stuck spacer.
- Shake the tube gently to allow cilia to spread out throughout the tube.
- Withdraw a small sample of ciliated epithelium in supplemented M199 from the middle of the tube with a pipette (approximately 60 μL) and fill the spacer.
- Stick the rectangular coverslip (22 mm x 40 mm) on the spacer to close the chamber.
- Disinfect the slide before getting out of the microbiological safety cabinet.
- Remove the slide from the microbiological safety cabinet.
- Change gloves when exiting the microbiological safety cabinet.
- Wait 10 minutes before turning off the microbiological safety cabinet after use (to make sure that the environment of the microbiological safety cabinet is sterile before closing the door).
- Place the slide in the plate of the heated box. Close the heated box with the lid.
- Add oil on the oil-immersion objective.
- Place the heated box on the stage of the microscope.
- Turn on the heated box and the lens heater.
NOTE: The lens heater must be turned on 1 hour before use. - Adjust the temperature settings of the heated box and the lens heater controllers according to values obtained at step 3.4.
- Wait 5 minutes (time required to rise the temperature of the sample up to 37 °C when using predetermined settings for both the heated box and the objective heater).
- Approach the objective to the slide until touching the coverslip with the tip of the lens.
5. Visualizing respiratory ciliated edges
- Fix the high-speed video camera onto the microscope, connect the camera to the computer, and turn on the camera.
- Turn on the computer.
- Connect the digital high speed videomicroscopy camera to the computer (so that the image viewed through the ocular lenses is projected onto the monitor) via the software.
- Open the software, and then Main Menu opens automatically (Figure 6A).
NOTE: The software is the program used in the laboratory for image acquisition and processing. The system allows video sequences to be recorded and played back at a reduced frame rate or frame by frame. It can be downloaded for free. - Open Camera (Figure 6A).
- When Camera enumeration filter appears, choose OK (Figure 6B).
- Select Refresh List; select the name of the camera; choose the Interface: Expert, then select Open (Figure 6C).
- On the camera control-line at the top of the docked dialog menu, select Live (Figure 6D).
- Choose Play to view the image and Stop to finish viewing (Figure 6D).
- Open the software, and then Main Menu opens automatically (Figure 6A).

Figure 6: Description of the use of the software: visualization of respiratory ciliated edges onto the monitor. (A) The Main Menu appears directly when opening the software. (B) Close the Camera Enumeration Filter. (C) Choose the camera and select Interface: Expert. (D) The live mode allows to visualize on the monitor the image seen through the microscope. Please click here to view a larger version of this figure.
- Adjust the camera acquisition setting (in the top right corner) (Figure 7).
- On Acquisition Settings choose Camera, then adjust the frame rate: Rate (Hz): 500 (see below) (Figure 7A).
- On Acquisition Settings choose Camera, then adjust the region of interest (ROI) (Figure 7A).
NOTE: The ROI is calculated using a graduated scale viewed with the x100 oil-immersion objective and projected onto the monitor, to define the number of pixels corresponding to 50 µm (as you want to record ciliated edges measuring approximately 50 µm (see below)). - On Acquisition Settings choose Record, then adjust the duration of the video and the total number of frames recorded (a 2 seconds duration, corresponds to 1000 frames if the frame rate chosen is 5OO Hz) (Figure 7B).
NOTE: In our experience, a minimum of 2 seconds video length is necessary to allow a complete analysis of both CBF and CBP. - Choose File then Save Camera Cfg to save the new acquisition setting (enter a name and if necessary a comment for this new configuration) (Figure 7C,D).
- To open this new camera configuration, open File and Load Camera Cfg (Figure 7C).
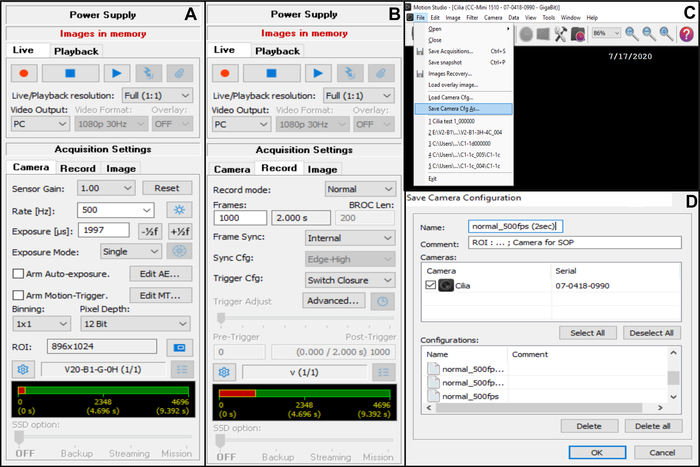
Figure 7: Description of the use of the software: adjustment of the camera acquisition settings for video recording of the beating ciliated edges. (A) On the acquisition setting Camera, adjust the region of interest (ROI) and frame rate for video recording (Rate). (B) On the acquisition setting Record, adjust the duration of the video recording (number of frames needed for the chosen recording duration, according to the frame rate chosen previously). (C) This new camera configuration settings can be saved using the Save camera Cfg function. Load Camera Cfg allows to reopen the saved configuration settings for further used. (D) The new camera configuration settings can be named, and a comment can be added if necessary. Please click here to view a larger version of this figure.
- View through ocular lenses and search for cells or debris within the sample, then focus.
- Check that the image is visible on the monitor, and improve the quality of the image by adjusting the condenser, (and the DIC prism if using an interference contrast lens), and adjust the focus if necessary.
- Search for strips of ciliated epithelium.
6. Respiratory ciliated edges selection
NOTE: The experimental system allows beating cilia to be viewed in three distinct planes: a sideways profile, beating directly towards the observer, and from directly above (Figure 8).
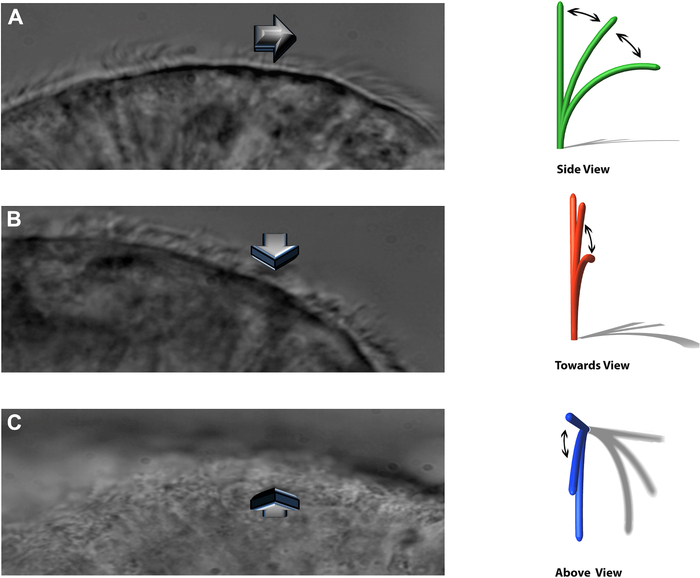
Figure 8: The DHSV technique allows beating cilia to be viewed in three distinct planes. (A) in the sideways profile. (B) beating directly towards the observer and. (C) from directly above. Reproduced from Kempeneers et al.16. Please click here to view a larger version of this figure.
- Record only intact undisrupted ciliated epithelial edges that measure at least 50 μm in length.
- For records made on the sideways profile, determine the quality of the edge according to Thomas et al.29 scoring system (Figure 9). Use only normal edges (Figure 9A) or edges with minor projections (Figure 9B) for ciliary functional analysis. Exclude isolated cells (Figure 9E).

Figure 9: Representative image of the scoring system by Thomas et al29 for the different quality of ciliated epithelial edges. (A) Normal edge: defined as an intact uniform ciliated epithelia strip > 50 μm in length (B) Ciliated edge with minor projections: defined as an edge >50 μm in length, with cells projecting out of the epithelial edge line, but with no point of the apical cell membrane projecting above the tips of the cilia on the adjacent cells (C) Ciliated edge with major projections: defined as an edge >50 μm in length, with cells projecting out of the epithelial edge line, with at least one point of the apical cell membrane projecting above the tips of the cilia on the adjacent cells (D) Isolated ciliated cell: defined as the only ciliated cell on an epithelial edge >50 μm in length (E) Single cells: defined as ciliated cells that have no contact between themselves or any other cell type. Scale bar: 5.5 μm. Reproduced from Thomas et al.29 Please click here to view a larger version of this figure.
- Perform CFA using only cilia free of mucus and debris, and beating in the profile chosen for the recorded edge. Select only ciliated edges that allow a minimum of 2 CBF and CBP evaluation (see below) along the edge.
- Use for CFA only samples that yield a minimum of 6 edges beating in the sideways profile and meeting the above criteria; analyze a maximum of 20 edges in the sideways profile.
- Use a minimum of 1 additional edge of cilia beating from above the observer profile to characterize the CBP.
7. Recording ciliated edge
- Record the beating cilia edge using a camera frame rate of 500 frames per second, and project onto a high-resolution monitor. A minimum frame rate of 400 Hz is required to allow the analysis of both CBF and CBP13. Record one edge at a frame rate of 30 frames per second to evaluate the efficiency of particulate clearance.
- Select Live, on the camera control-line at the top of the docked dialog menu (Figure 6D)
- Choose Play to view the image and Stop to finish viewing (Figure 6D)
- To record an edge, press Record (Figure 6D). To view the recording before saving, go on camera control-line at the top of the docked dialog menu and select Playback. Choose Play to view the video recorded and Stop to finish viewing (Figure 10A).
NOTE: Stop viewing the recorded edge before saving.
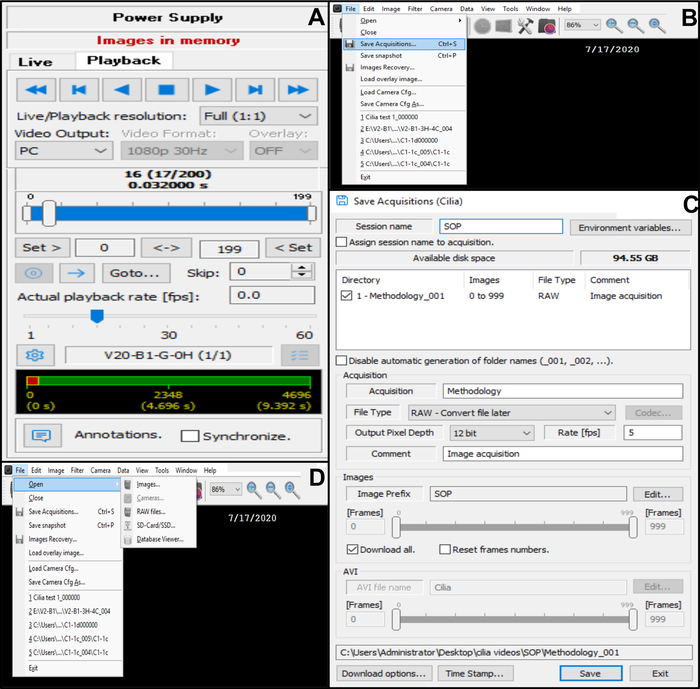
Figure 10: Description of the use of the software. (A) playback mode. To review a recorded video sequence of beating ciliated edge, choose the Playback Mode. Choose Play to view the image and Stop to finish viewing. The fame rate can be adjusted to improve the analysis of ciliary function (B, C) Saving the video recordings of beating ciliated edges (B) To save the video, choose File then Save Acquisitions. (C) Enter the name of the recorded video and choose the emplacement where the video is recorded. Make sure that the recording is saved as a .RAW file (D) choice of a recording of beating ciliated edges to be analyzed: To open a video recording, choose File, then Open, then Images. Please click here to view a larger version of this figure.
- Save the video in the database (Figure 10B,C).
- Open File in the top left corner, then save acquisitions (Figure 10B).
- In Save acquisitions, enter the name of the recorded video and make sure that the recording is saved as a RAW file type format (Figure 10C).
- When the video is saved, return to the live mode (go back to the camera control-line at the top of the docked dialog menu and select live) (Figure 6D).
- Repeat the procedure to record the number of edges meeting the selection criteria required for CFA.
NOTE: It is possible to record several beating ciliated edges meeting the selection criteria from one slide, within a maximum of 20 minutes after the preparation of the slide (to avoid desiccation). After 20 minutes, if it is not possible to obtain enough edges meeting the selection criteria, prepare a new a slide. - Remove the slide from the heated box.
- Remove the rectangular coverslip and throw it in the specific hazardous medical waste container.
- Clean the slide (with the two squared cover slips glued on it) with 70% ethanol and absorbent paper. Once the slide is clean, it can be used again.
- COVID-19 adaptation: Place the slide with the coverslip and spacer in an airtight bag, remove gloves and mask and place them in the airtight bag. Place the airtight bag in the specific hazardous medical waste container.
8. Ciliary functional analysis
- Preliminary preparation to perform the manual CBF and CBP evaluation
- Open the software.
- Open File in the top left corner, then Open and then Images (Figure 10D).
- Choose the video to analyze.
- Go on camera control-line at the top of the docked dialog menu and select Playback (Figure 10A). Choose Play to view the video recorded and Stop to finish viewing.
- Manual ciliary beat frequency (CBF) analysis
- Perform the evaluation of CBF using the sideways edges only.
- Divide the ciliated edges into approximatively 5 adjacent areas, each measuring approximatively 10 μm (Figure 11).
- Identify and visualize cilia or groups of cilia at a reduced frame rate, and a maximum of 2 CBF measurements are made in each area, resulting in a maximum of 10 CBF measurements along each edge (Figure 11).
- Record the number of frames required for a group of cilia to complete 5 beat cycles.
- Convert to CBF by a simple calculation: (CBF= recording frame rate (Hz)/(number of frames for 5 beats) x 5)13,16,30. Immotile cilia are reported as having a CBF of 0 Hz13.
NOTE: Adjust the frame rate when playing back the recorded videos (Figure 10A). This is especially useful when the cilia analyzed beat very slowly. Increasing the frame rate helps to define if the cilia beat very slowly or are immotile. - For each sample, calculate the mean CBF as the mean (SD) or (95% CI) of all CBF recorded in the sideways profile, including static cilia.
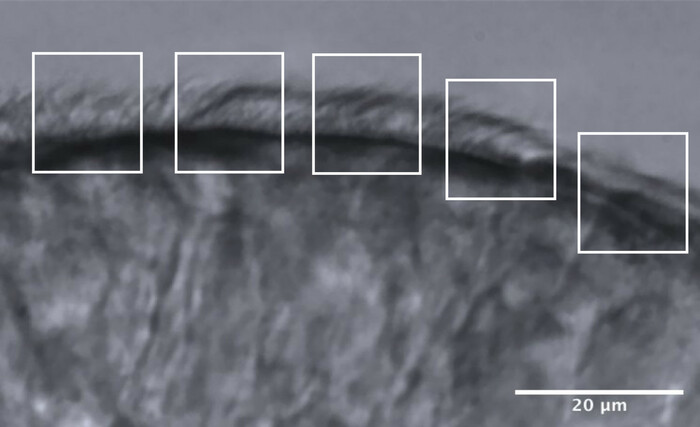
Figure 11: Representative image of an optimal quality edge, and the division into 5 areas to allow CFA analysis. An optimal quality ciliated epithelial edge is fragmented into 5 adjacent areas each measuring 10 μm. A maximum of 2 CBF measurements (and 2 CBP evaluation) are made in each area, resulting in a maximum of 10 CBF measurements (and CBP evaluations) along each edge. Scale bar = 20 μm. Please click here to view a larger version of this figure.
- Manual ciliary beat pattern (CBP) analysis
- To evaluate the markers of dyskinesia, use the sideway profile only; use the planes towards the observer and from above to characterize the type of CBP13. Different methods and scores for CBP evaluation exist. Below is described the method used in the laboratory with the definition of the markers of dyskinesia.
- The percentage of each distinct CBP within the sample
- For each cilia or group of cilia identified and used for a CBF measurement (Figure 11), perform a CBP analysis at a reduced frame rate: compare the precise path taken by the cilia during a full beat cycle with the normal CBP observed on the DHSV analysis12,30.
- Attribute a distinct CBP (normal, immotile, stiff, circular, asynchronous (uncoordinated ciliary beating) or dyskinetic13) to each cilia or group of cilia analyzed.
- For each sample, calculate the percentage of each distinct CBP within the sample; the CBP attributed to the sample is the predominant CBP observed.
- Calculate the 3 markers of dyskinesia.
- Calculate the immotility index (IMI): the percentage of immotile cilia within the sample (number of CBF=0/total number of CBF readings in the sample X 100). Express the IMI as mean (SD) or (95% CI)1,16,31.
- Calculate the dyskinesia score (DKS). Divide each ciliated edge into quadrants, and the number of quadrants with dyskinetic (or abnormally beating) cilia is determined. This allows a DKS between 0 and 4 to be calculated (0: normal CBP throughout the edge; 1: abnormal CBP in ≤ 25% of cilia; 2: abnormal CBP in ≤ 50% of cilia; 3: abnormal beat pattern in ≤ 75% of cilia; and 4: abnormal CBP in all cilia). The median DKS (interquartile range) is calculated for the sample16,29.
- Calculate the percentage of normal beating: defined as the percentage of cilia with a normal CBP within the sample (number of normal CBP readings/total number of CBP readings for the sample x100).
Results
To illustrate the efficiency of the technique, we present the results of the CFA in a series of 16 healthy adult volunteers (5 males, age range 22-54 years).
Nasal brushing samples from 14 (4 males, age range 24-54 years) out of the total of 16 volunteers provided enough appropriate epithelial edges that satisfied the selection criteria needed to perform CFA. From these 14 nasal brushing samples, a total of 242 ciliated edges were recorded, and 212 edges met the defined inclusion criteria and ...
Discussion
This paper aims to provide a standard operating procedure for CFA using nasal brushing samples, with adjustments made for appropriate infection control considerations during the COVID-19 pandemic. PCD diagnosis is challenging, and currently requires a panel of different diagnostic tests, according to international recommendation, including nasal nitric oxide measurement, CFA using DHSV, ciliary ultrastructural analysis using transmission electron microscopy (TEM), labelling of ciliary proteins using immunofluorescence, a...
Disclosures
These authors have nothing to disclose.
Acknowledgements
We would like to thank Jean-François Papon, Bruno Louis, Estelle Escudier and all team members of PCD diagnostic center of Paris-Est for their availability and hearty welcome during the visit to their PCD diagnostic center, and the numerous exchanges. We also thank Robert Hirst and all team members at the PCD center of Leicester for their welcome and time, advice, and expertise.
Materials
| Name | Company | Catalog Number | Comments |
| 15 mL conical tubes | FisherScientific | 352096 | 15 ml High-Clarity Polypropylene Conical Tube with lid |
| Amphotericin B | LONZA | 17-836E | Antifungal solution |
| Blakesley-weil nasal forceps | NOVO SURGICAL | E7739-12 | Used to hold the brush to perform the nasal brushing |
| Bronchial cytology brush | CONMED | 129 | Used for nasal brushing |
| Cotton swab | NUOVA APTACA | 2150/SG | Used for COVID-19 testing |
| Digitial high-speed videomicroscopy camera | IDTeu Innovation in motion | CrashCam Mini 1510 | |
| Glass slide | ThermoScientific | 12372098 | Microscope slides used to create the visualization chamber |
| Heated Box | IBIDI cells in focus | 10918 | Used to heat the sample |
| Inverted Light microscope | Zeiss | AXIO Vert.A1 | |
| Lens Heater | TOKAI HIT | TPiE-LH | Used to heat the oil immersion lens |
| Medium 199 (M199), HEPES | TermoFisher Scientific | 12340030 | Cell Culture Medium |
| Motion Studio X64 | IDT Motion | version 2.14.01 | Software |
| Oil | FischerScientific, Carl Zeiss | 11825153 | |
| Rectangular cover slip | VWR | 631-0145 | Used to cover the visualization chamber |
| Spacer (Ispacer) 0.25 mm | Sunjinlab | IS203 | Used for the creation of the hermetic closed visualization chamber |
| Square cover slip | VWR | 631-0122 | Used for the creation of lab-built open visualization chamber |
| Streptomycin/Penicillin | FisherScientific, Gibco | 11548876 | Antiobiotics solution |
References
- Chilvers, M. A., Rutman, A., O'Callaghan, C. Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. Journal of Allergy Clinical Immunology. 112 (3), 518-524 (2003).
- Werner, C., Onnebrink, J. G., Omran, H. Diagnosis and management of primary ciliary dyskinesia. Cilia. , 1-9 (2015).
- Kempeneers, C., Chilvers, M. A. To beat, or not to beat, that is question! The spectrum of ciliopathies. Pediatric Pulmonology. 53 (8), 1122 (2018).
- Lucas, J. S., et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. The European Respiratory Journal. 49 (1), (2017).
- Knowles, M. R., Zariwala, M., Leigh, M. Primary Ciliary Dyskinesia. Clinics in chest medicine. 37 (3), 449-461 (2016).
- Shapiro, A. J., et al. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatric Pulmonology. , (2016).
- Fitzgerald, D. A., Shapiro, A. J. When to suspect primary ciliary dyskinesia in children. Paediatric Respiratory Reviews. , (2016).
- Shoemark, A., Dell, S., Shapiro, A., Lucas, J. S. ERS and ATS diagnostic guidelines for primary ciliary dyskinesia: similarities and differences in approach to diagnosis. European Respiratory Journal. 54 (3), (2019).
- Mirra, V., Werner, C., Santamaria, F. Primary ciliary dyskinesia: An update on clinical aspects, genetics, diagnosis, and future treatment strategies. Frontiers in Pediatrics. 5, 1-13 (2017).
- Ardura-Garcia, C., et al. Registries and collaborative studies for primary ciliary dyskinesia in Europe. European Respiratory Journal Open Research. 6 (2), (2020).
- Leigh, M. W., et al. Clinical features and associated likelihood of primary ciliary dyskinesia in children and adolescents. Annals of the American Thoracic Society. , (2016).
- Chilvers, M. A., O'Callaghan, C. Analysis of ciliary beat pattern and beat frequency using digital high speed imaging: comparison with the photomultiplier and photodiode methods. Thorax. 55 (4), 314-317 (2000).
- Kempeneers, C., Seaton, C., Garcia Espinosa, B., Chilvers, M. A. Ciliary functional analysis: Beating a path towards standardization. Pediatric Pulmonology. 54 (10), 1627-1638 (2019).
- Barbato, A., et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. The European respiratory journal. 34 (6), 1264-1276 (2009).
- Raidt, J., et al. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. European Respiratory Journal. 44 (6), 1579-1588 (2014).
- Kempeneers, C., Seaton, C., Chilvers, M. A. Variation of Ciliary Beat Pattern in Three Different Beating Planes in Healthy Subjects. Chest. 151 (5), 993-1001 (2017).
- Götzinger, F., et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. The Lancet Child & Adolescent Health. , (2020).
- Yang, J., et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta-analysis. International Journal of Infectious Diseases. 94, 91-95 (2020).
- Brough, H. A., et al. Managing childhood allergies and immunodeficiencies during respiratory virus epidemics - The 2020 COVID-19 pandemic: A statement from the EAACI-section on pediatrics. Pediatric Allergy and Immunology. 31 (5), 442-448 (2020).
- Zou, L., et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. The New England journal of medicine. 382 (12), 1177-1179 (2020).
- van Doremalen, N., et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. The New England journal of medicine. 382 (16), 1564-1567 (2020).
- Tran, K., Cimon, K., Severn, M., Pessoa-Silva, C. L., Conly, J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PloS one. 7 (4), 35797 (2012).
- Van Gerven, L., et al. Personal protection and delivery of rhinologic and endoscopic skull base procedures during the COVID-19 outbreak. Rhinology. 58 (3), 289-294 (2020).
- Marty, F. M., Chen, K., Verrill, K. A. How to Obtain a Nasopharyngeal Swab Specimen. New England Journal of Medicine. 382 (22), 76 (2020).
- Petruzzi, G., et al. COVID-19: Nasal and oropharyngeal swab. Head & Neck. 42, (2020).
- George, A., Prince, M., Coulson, C. Safe nasendoscopy assisted procedure in the post-COVID-19 pandemic era. Clinical Otolaryngology. , (2020).
- Hirst, R. A., et al. Culture of primary ciliary dyskinesia epithelial cells at air-liquid interface can alter ciliary phenotype but remains a robust and informative diagnostic aid. PLoS ONE. 9 (2), (2014).
- Jorissen, M., Willems, T., Van der Schueren, B. Ciliary function analysis for the diagnosis of primary ciliary dyskinesia: advantages of ciliogenesis in culture. Acta oto-laryngologica. 120 (2), 291-295 (2000).
- Thomas, B., Rutman, A., O'Callaghan, C. Disrupted ciliated epithelium shows slower ciliary beat frequency and increased dyskinesia. European Respiratory Journal. 34 (2), 401-404 (2009).
- Chilvers, M. A., Rutman, A., O'Callaghan, C. Functional analysis of cilia and ciliated epithelial ultrastructure in healthy children and young adults. Thorax. 58 (4), 333-338 (2003).
- Stannard, W. A., Chilvers, M. A., Rutman, A. R., Williams, C. D., O'Callaghan, C. Diagnostic testing of patients suspected of primary ciliary dyskinesia. American Journal of Respiratory and Critical Care Medicine. 181 (4), 307-314 (2010).
- Boon, M., et al. Primary ciliary dyskinesia: critical evaluation of clinical symptoms and diagnosis in patients with normal and abnormal ultrastructure. Orphanet Journal of Rare Diseases. 9 (1), 11 (2014).
- Armengot, M., Milara, J., Mata, M., Carda, C., Cortijo, J. Cilia motility and structure in primary and secondary ciliary dyskinesia. American Journal of Rhinology & Allergy. 24 (3), 175-180 (2010).
- Papon, J. F., et al. Quantitative analysis of ciliary beating in primary ciliary dyskinesia: a pilot study. Orphanet Journal of Rare Diseases. 7 (1), 78 (2012).
- Wallmeier, J., et al. Mutations in CCNO and MCIDAS lead to a mucociliary clearance disorder due to reduced generation of multiple motile cilia. Molecular and Cellular Pediatrics. 2, 15 (2015).
- Boon, M., et al. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nature Communications. 5 (6), 4418 (2014).
- Shapiro, A. J., et al. Diagnosis of Primary Ciliary Dyskinesia. An Official American Thoracic Society Clinical Practice Guideline. American Journal of Respiratory and Critical Care Medicine. 197 (12), 24-39 (2018).
- Rubbo, B., et al. Accuracy of high-speed video analysis to diagnose primary ciliary dyskinesia. Chest. (19), 30205 (2019).
- Horani, A., Ferkol, T. W. Advances in the Genetics of Primary Ciliary Dyskinesia. Chest. 154 (3), 645-652 (2018).
- MacCormick, J., Robb, I., Kovesi, T., Carpenter, B. Optimal biopsy techniques in the diagnosis of primary ciliary dyskinesia. The Journal of Otolaryngology. 31 (1), 13-17 (2002).
- Jackson, C. L., et al. Accuracy of diagnostic testing in primary ciliary dyskinesia. European Respiratory Journal. 47 (3), 837-848 (2016).
- Jackson, C. L., Goggin, P. M., Lucas, J. S. Ciliary Beat Pattern Analysis Below 37°C May Increase Risk of Primary Ciliary Dyskinesia Misdiagnosis. Chest. 142 (2), 543-544 (2012).
- Green, A., Smallman, L. A., Logan, A. C., Drake-Lee, A. B. The effect of temperature on nasal ciliary beat frequency. Clinical otolaryngology and allied sciences. 20 (2), 178-180 (1995).
- Clary-Meinesz, C. F., Cosson, J., Huitorel, P., Blaive, B. Temperature effect on the ciliary beat frequency of human nasal and tracheal ciliated cells. Biology of the Cell. 76 (3), 335-338 (1992).
- Smith, C. M., et al. ciliaFA: a research tool for automated, high-throughput measurement of ciliary beat frequency using freely available software. Cilia. 1 (1), 14 (2012).
- Sisson, J. H., Stoner, J. a., Ammons, B. a., Wyatt, T. a. All-digital image capture and whole-field analysis of ciliary beat frequency. Journal of Microscopy. 211, 103-111 (2003).
- Blanchon, S., et al. Deep phenotyping, including quantitative ciliary beating parameters, and extensive genotyping in primary ciliary dyskinesia. Journal of Medical Genetics. , (2019).
- Feriani, L., et al. Assessing the Collective Dynamics of Motile Cilia in Cultures of Human Airway Cells by Multiscale DDM. Biophysical Journal. 113 (1), 109-119 (2017).
- Sears, P. R., Thompson, K., Knowles, M. R., Davis, C. W. Human airway ciliary dynamics. American Journal of Physiology - Lung Cellular and Molecular Physiology. 304 (3), 170-183 (2013).
- Quinn, S. P., et al. Automated identification of abnormal respiratory ciliary motion in nasal biopsies. Science translational medicine. 7 (299), (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved