Cardiac Spheroids as in vitro Bioengineered Heart Tissues to Study Human Heart Pathophysiology
In This Article
Summary
This protocol aims to fabricate 3D cardiac spheroids (CSs) by co-culturing cells in hanging drops. Collagen-embedded CSs are treated with doxorubicin (DOX, a cardiotoxic agent) at physiological concentrations to model heart failure. In vitro testing using DOX-treated CSs may be used to identify novel therapies for heart failure patients.
Abstract
Despite several advances in cardiac tissue engineering, one of the major challenges to overcome remains the generation of a fully functional vascular network comprising several levels of complexity to provide oxygen and nutrients within bioengineered heart tissues. Our laboratory has developed a three-dimensional in vitro model of the human heart, known as the "cardiac spheroid" or "CS". This presents biochemical, physiological, and pharmacological features typical of the human heart and is generated by co-culturing its three major cell types, such as cardiac myocytes, endothelial cells, and fibroblasts. Human induced pluripotent stem cells-derived cardiomyocytes (hiPSC-CMs or iCMs) are co-cultured at ratios approximating the ones found in vivo with human cardiac fibroblasts (HCFs) and human coronary artery endothelial cells (HCAECs) in hanging drop culture plates for three to four days. The confocal analysis of CSs stained with antibodies against cardiac Troponin T, CD31 and vimentin (markers for cardiac myocytes, endothelial cells and fibroblasts, respectively) shows that CSs present a complex endothelial cell network, resembling the native one found in the human heart. This is confirmed by the 3D rendering analysis of these confocal images. CSs also present extracellular matrix (ECM) proteins typical of the human heart, such as collagen type IV, laminin and fibronectin. Finally, CSs present a contractile activity measured as syncytial contractility closer to the one typical of the human heart compared to CSs that contain iCMs only. When treated with a cardiotoxic anti-cancer agent, such as doxorubicin (DOX, used to treat leukemia, lymphoma and breast cancer), the viability of DOX-treated CSs is significantly reduced at 10 µM genetic and chemical inhibition of endothelial nitric oxide synthase, a downstream target of DOX in HCFs and HCAECs, reduced its toxicity in CSs. Given these unique features, CSs are currently used as in vitro models to study heart biochemistry, pathophysiology, and pharmacology.
Introduction
The human heart has a limited regenerative capacity while cardiovascular disease (CVD) remains the main cause of death worldwide despite the recent advances in tissue engineering and stem cell technologies1. The need for novel therapeutics including molecular and cellular approaches to either repair a damaged heart or to prevent a heart from failing is one of the major current clinical needs for patients suffering from heart disease2,3,4. The main goal of cardiac tissue engineering is to fabricate a three-dimensional (3D) heart tissue that presents molecular, cellular, and extracellular features typical of a human heart, including its vascular network and physiological contractile function4,5,6.
In order to bioengineer and fabricate a functional human cardiac tissue that mimics the human heart for in vitro and in vivo applications, several approaches have been investigated including engineered heart tissues (EHTs), cell sheets and spheroid cultures7,8. However, these tissues fail at recapitulating the optimal 3D microenvironment typical of the human heart and their potential use for CVD patients cannot directly translate from the bench to the bedside7. This is because they do not recapitulate the complex biology, morphology, and physiology of in vivo heart tissues9. One of the major challenges in cardiac tissue engineering includes the development of a hierarchical vascular network within the bioengineered cardiac tissue, as any tissue that is bigger than 200 μm in diameter develops cell death in the middle2,10. A properly formed vascular network in a human heart tissue plays an major role for the supply of blood, oxygen and nutrients to cardiac cells11. During embryonic development, coronary capillaries and arteries form via vasculogenesis (de novo blood vessel formation) and angiogenesis (generation of blood vessels from pre-existing ones) from endothelial progenitor cells8,12. Cardiac fibroblasts also play a major role in proper vascular network formation by providing the optimal extracellular matrix (ECM) and growth composition13,14.
The 3D vascular network of bioengineered heart tissues controls cell survival and function by creating oxygen and nutrient gradients and paracrine signaling, such as homotypic cell interaction, heterotypic cell interaction, interaction of cells through secreted soluble proteins and cell to ECM interactions3,10,15,16,17,18. This prevents cell death in the middle of the tissue and promotes cell viability and physiological function in bioengineered heart tissues16,18,19.
Spheroid cultures from stem cells have been recently explored as in vitro models of the human heart20. To further improve the cardiac microenvironment in vitro, they have included the use of all the main cell types found in the human heart, such as cardiac myocytes, endothelial cells, and fibroblasts. Spheroid cultures present the required 3D structural support for cells to grow and function and can be used to bioengineer a vascular network14,20,21,22. In this context, our laboratory has developed human cardiac spheroids (CSs) by co-culturing cardiac myocytes, endothelial cells and fibroblasts at ratios found in the human heart14. This model is an expansion of the rat ventricular cardiac cells spheroid model, generated by co-culturing cardiac cells in hanging drop cultures, used to model cardiac fibrosis21. Human CSs can be used as toxicity assays by treating them doxorubicin (DOX, an anti-cancer agent used to treat leukemia, lymphoma and breast cancer), which is well-known to induce cardiac fibrosis and heart failure (HF) even 17 years following its somministration14.
In this manuscript, we describe how to generate human CSs by co-culturing human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs or iCMs), human cardiac fibroblasts (HCFs) and human coronary artery endothelial cells (HCAECs) in hanging drop cultures. In order to use and image CSs for in vitro testing, they are embedded in a collagen gel. The confocal analysis of CSs stained with antibodies against CD31, a marker for endothelial cells, showed that these cells form a network similar to the one observed in vivo. To induce HF and potentially test novel agents that may treat or prevent it, CSs were treated with 10 µM DOX (a concentration found in the bloodstream of cancer patients receiving the drug). When stained with calcein-AM and ethidium homodimer (staining live and dead cells, respectively), DOX-treated CSs present a significant decrease in viability in comparison to CSs that did not receive the drug. CSs also present a homogeneous contractile activity when paced using field potential stimulation between 1 and 3 Hz.
Protocol
NOTE: hiPSC-CMs used for this protocol are commercially available. Please seek institutional human ethics committee approval before commencing this work if required.
1. Human cardiac fibroblast and endothelial cell culture plating and growth
- Thaw cryovials containing HCFs and HCAECs in a water bath at 37 °C for one minute.
- Move cryovials under a sterile laminar flow biosafety cabinet class 2.
- Collect 1 mL of cell suspension from the cryovials using a 1000 µL pipette tip and add into a 15 mL tube containing 7 mL of Human Cardiac Fibroblast Medium for HCFs and 7 mL of Human Meso Endo Growth Medium for HCAECs.
NOTE: In order to collect the majority of the cells from each cryovial, rinse them twice with 1 mL of culture medium from the same 15 mL tube. - Gently mix cell suspensions.
- Transfer cell suspensions to separate T75 culture flasks using a 10 mL serological pipette.
- Incubate cells at 37 °C with 5% CO2.
- After 18 h, aspirate the medium from both culture flasks and rinse them once with sterile phosphate buffered saline (PBS) to remove freezing medium and dead cells.
- Replace PBS with 7 mL of appropriate culture medium to each culture flask and incubate at 37 °C.
- Examine cellular expansion and viability regularly and replace media every other day until cells reach 80-90% confluency.
2. iCM culture plating and growth
- Pre-coat two T25 culture flasks with 2 mL of PBS containing 40 µg/mL of fibronectin (FN) and incubate at 37 °C, 5% CO2 for at least 4 hours.
- After 4 hours, collect one cryovial containing iCMs and place it in a water bath at 37 °C for 4 min.
- Move the cryovial under a sterile laminar flow biosafety cabinet class 2.
- Gently transfer the iCMs from the cryovial to a sterile 50 mL centrifuge tube using a 1 mL pipette tip.
- Rinse the empty iCMs cryovial with 1 mL of room temperature plating medium to recover any residual cells. Transfer the 1 mL of plating medium rinse from the cryovial drop-wise over 90 sec to the 50 mL centrifuge tube containing the iCM cell suspension.
NOTE: Gently swirl the tube while adding the medium to mix the solution completely and to decrease the osmotic shock on the thawed cells. - Slowly add 8 mL of room temperature Plating Medium to the 50 mL centrifuge tube. Add the first 1 mL dropwise over 30 - 60 s. Then, add the remaining volume over the next 30 s. Gently swirl the centrifuge tube while adding the Plating medium. Gently mix the contents of the 50 mL centrifuge tube by inverting 2 - 3 times (avoiding vigorous shaking or vortexing).
- Immediately perform the cell counting using a hemocytometer and determine the viable cell density (in cells/mL).
- Take the FN-pre-coated T25 flasks and aspirate the FN-PBS solution without letting the flasks dry. To this add seeding volume of iCMs (1.6 x 106 viable iCMs in 8 mL room temperature plating medium).
- Culture iCMs in the incubator for 48 h at 37 °C, 5% CO2.
- Thaw the Maintenance Medium overnight at 4 °C a day before use.
- Equilibrate the Maintenance Medium in a 37 °C water bath and use immediately.
- After 2 days, move the iCMs T25 flasks under the biosafety cabinet.
- Gently wash off dead cells and debris by gently pipetting the Plating Medium up and down 5 times.
- Aspirate the Plating Medium and replace with the 8 mL of pre-warmed Maintenance Medium. Place the T25 flasks back in the incubator. Replace the Maintenance Medium every other day and examine the confluency regularly.
3. Cell isolation and counting
- Start by collecting first HCAECs and HCFs, and then iCMs by following steps 3.2-3.12.
- Prepare CS culture medium by mixing 10 mL of iCMs Maintenance Medium, 5 mL of Human Cardiac Fibroblast Medium and 5 mL of Meso Endo Growth Medium.
- Remove culture medium from each tissue flask containing HCFs and HCAECs and rinse once with 5 mL PBS for T75 flasks. Remove PBS.
- Add 5 mL of 0.25% trypsin EDTA solution to each T75 flask and incubate for 5 min at 37 °C, 5% CO2.
- Once cells detach, immediately neutralize the trypsin EDTA solution with 5 mL of culture medium.
- Transfer cell suspensions to a 15 mL tube and centrifuge cells at 300 x g for 4 min.
- Remove the supernatant carefully from each tube. Add 1 mL of CS medium to each cell pellet and resuspend them. Keep the tube on ice and count cells using Trypan Blue and a hemocytometer.
- Remove Maintenance Medium from tissue flasks containing iCMs and rinse once with 3 mL of PBS.
- Add 1 mL of 0.25% trypsin EDTA solution to each T75 flask and incubate at 37 °C, 5% CO2. Check cells every minute until detached.
- Once cells detach, immediately neutralize the trypsin EDTA solution with 4 mL of culture medium.
- Transfer cell suspensions to a 15 mL tube and centrifuge them at 300 x g for 5 min.
- Remove the supernatant carefully from each tube. Add 1 mL of culture medium to the cell pellet and resuspend it. Keep the tube on ice and count cells using Trypan Blue and a hemocytometer.
4. CS generation and growth
- Mix iCMs, HCFs and HCAECs in 2:1:1 ratio by plating 10,000 iCMs, 5,000 HCFs and 5,000 HCAECs per hanging drop culture containing 20 µL of CS medium. Adjust to the final volume for the total number of CSs.
- Pipette 20 µL of cell suspension into each well of the 384 well HDC plate either manually or by using a robotic multichannel pipette for automated liquid handling.
- Pipette 1.5 mL of sterile PBS in each side of the channel around the Hanging Drop Plate to prevent drying out CSs. Incubate HDC plate at 37 °C.
- Examine formation of CSs on a daily basis until a fully formed spheroid is observed in the majority of wells. Add 7.5 µL of CS medium to each well every other day until a CS is formed.
5. CS embedding in collagen gels
- Collect CSs with a 1 mL pipette tip.
NOTE: It is necessary to cut the tip of pipette around 0.2 cm from the edge before its use with a sterile sharp surface (either a scalpel or a scissor) to prevent any damage gel embedded spheroids during their collection. - Collect the CS suspension into a 50 mL tube on ice.
- Centrifuge the tube at 300 x g for 5 min.
NOTE: the pellet obtained must be kept on ice until use. - Prepare a collagen gel solution (100 µL/well for 30 wells of 96 well plate) on ice using rat tail collagen and CS medium in a 3:7 ratio.
- Remove the supernatant from the tube containing CSs.
- Mix the pelleted CSs within the collagen gel solution.
- Add 1 µL/mL of 5 mM sodium hydroxide to the CS-collagen gel suspension and mix gently.
- Transfer 100 µL of CS-collagen gel to a clear flat bottom 96 well black polystyrene microplate and incubate at 37 °C for 30 min.
6. Viability and toxicity measurements of DOX-treated CSs
- After 30 min collect the 96 well plate from incubator
- Prepare a 10 µM DOX (based on previously established protocol for cell death in CSs14).
NOTE: To potentially test other agents that may protect against HF in CSs, generate solutions containing DOX + Agent A, B, etc. - Add 100 µL of solutions containing DOX and/or other agents to each well. Control cultures contain media without any DOX.
- Incubate plate for 18 h at 37 °C, 5% CO2.
- On the following day, collect the Live/Dead staining reagent stock solutions and allow them to thaw on ice in the dark in a biosafety cabinet.
- Prepare a solution containing Hoechst stain, 4 µM of ethidium homodimer and 2 µM calcein-AM.
- Add 100 µL of Hoechst stain, calcein-AM/ethidium homodimer solution into each well.
- Measure the fluorescence into each well at 645 nm for ethidium homodimer and at 530 nm for calcein-AM, respectively, using multimode microplate reader.
- Transfer fluorescence measurements into Graphpad PRISM (or an equivalent software for statistical analysis).
- Use GraphPad Prism software for data analysis and statistics.
- For quality control, check under an epifluorescence microscope for the nuclei staining, together with calcein-AM and ethidium homodimer.
7. CS contractile function evaluation
- Collect the microplate as prepared in step 5.8.
- Turn the on the computer containing the IonOptix software for a video-based edge-detection, the Fluorescence Assistance Interface, and the MyoPacer Field Stimulator.
- Place a new cover slip on the tissue holder platform and assemble water bath with electrodes.
- Gently collect CSs from collagen gels using a 1 mL pipette tip cut 0.5 mm from the edge and transfer them to a falcon tube. Add media onto CS to prevent drying of CSs. Transfer CS (one at a time) with a few µL of media on the stage of the IonOptix system.
- Select the CS to be analysed via setting peaks on left and right side of CS using the IonOptix software.
- Use the computer-based motion analyzer to track the movement of CS edges.
NOTE: Normally, contractility is measured in either % cell shortening or % fractional shortening. In this case, we measured % spheroid shortening. - Stabilize both the peaks adjusting threshold and edge options from the computer.
- Expose CSs to different frequencies (0.3, 0.6, 1, 2 and 3 Hz) and voltages (1, 2, 3 and 5 V) using the Myopacer Field Stimulator.
- Record spheroid shortening as CS length changes of DOX-treated and untreated CSs. Analyse data using the Soft-Edge software and averaged for each CS.
8. Microscopy of CSs: fixation and immunolabeling
- Collect the 96 well plate after 30 min (as prepared in step 5.8) and fix CSs in 4% paraformaldehyde (PFA) for 1 h at room temperature.
- Remove PFA and rinse three times with PBS containing 0.01% sodium azide (PBSA).
- Remove PBSA.
- Add 200 µL of PBSA containing 0.02% Triton-X-100 to each well for 30 min on a shaker.
NOTE: This step permeabilizes CSs for better antibody infiltration. - Add 200 µL of 3% bovine serum albumin in PBSA solution for 60 min at room temperature.
NOTE: This step blocks unspecific antibody binding in CSs. - Prepare a solution containing 10 µg/mL of primary mouse anti-human antibodies against CD31 diluted in blocking solution.
- Add 100 µL of primary antibody solution to each well and incubate overnight at 4 °C on a shaker.
- Rinse the plate three times with PBSA for 20 min at room temperature on a rocking plate.
- Prepare a solution containing Hoechst DNA stain and 10 µg/mL of Cy3-conjugated secondary donkey anti-mouse antibody diluted in blocking solution.
- Add 100 µL of secondary antibody solution containing Hoechst stain to each well and incubate overnight at 4 °C on a shaker.
NOTE: Cover the plate with aluminium foil from this point onwards. - Rinse the plate three times for 20 min with PBSA at room temperature on a rocking plate.
- Add 100 µL of Vectashield mounting medium to each well.
- Image CSs under a laser scanning confocal microscope. Perform optical sectioning along the Z axis and collapse images into a single focal plane using ImageJ software.
Representative Results
The protocol described in this manuscript represents an alternative approach to develop complex cardiac endothelial cell network within a bioengineered cardiac tissue with improved cell viability and function compared to existing models (Figure 1). The recapitulation of the 3D in vivo heart microenvironment within CSs promoted their response to DOX at the concentration found in the bloodstream of cancer patients (between 5 and 10 μM, Figure 2). DOX treated CSs presented a statistically significant reduction in cell viability compared with control (no DOX) CSs within 24 h (Figure 2), a toxic effect that is observed in human cancer patients even 17 years after their treatment with the drug.
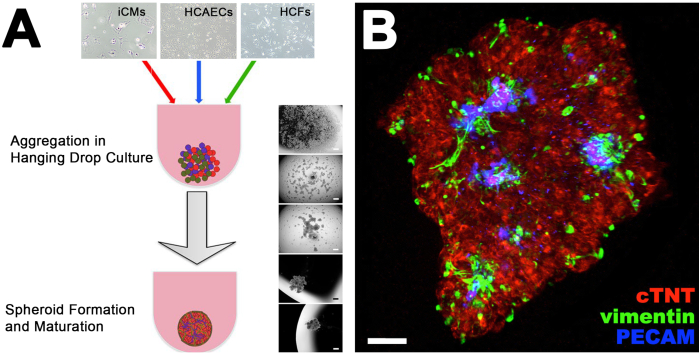
Figure 1. CS Formation and Vascularization Analysis. (A) Protocol showing the steps for the formation of a CS from the co-culture of iCMs, HCAECs and HCFs in hanging drops. Brightfield images on the right side show the progressive spheroid formation from single cells in hanging drops. (B) Collapsed Z-stacks of confocal images of a CS stained with antibodies against cardiac Troponin T (cTNT), PECAM and vimentin, staining cardiac myocytes, endothelial cells and fibroblasts, respectively. This figure has been modified from14. Please click here to view a larger version of this figure.
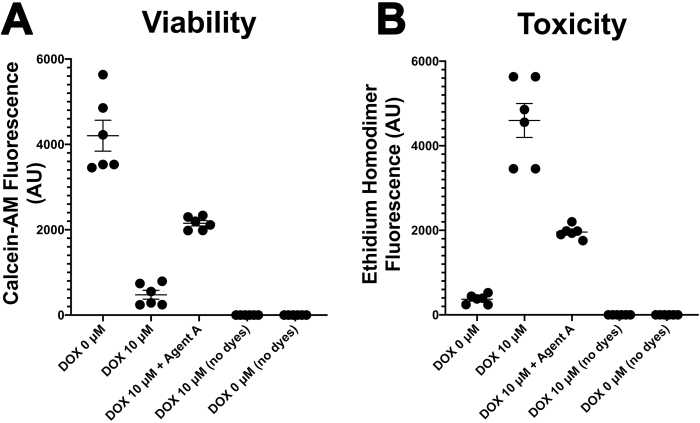
Figure 2. Viability and Toxicity in CSs. Statistical analyses of calcein-AM (A) and ethidium homodimer (B) fluorescence of CSs treated in presence of either media only (DOX 0 μM) or doxorubicin (10 μM). Please click here to view a larger version of this figure.
Discussion
Developmentally, proper vascular network formation is critical for the generation of functional tissues, including the human heart10,12,23,24,25,26. Consideration for the proper vascularization of 3D tissues allow the exchange of oxygen, growth factors, signalling molecules and nutrients, preventing the development of cell necrosis within any tissue thicker than 200 μm6,10,12,17,24,25,26,27,28. Currently available in vitro 3D heart models that present a vascular network are primarily presenting capillary-sized, disorganized vascular networks and lack the hierarchical complex branched vascularization observed in vivo6,8,29. The alternative approach to develop complex cardiac endothelial cell network described in this manuscript presents improved cell viability and function compared to existing models (Figure 1)14,22. 3D in vitro CSs model the human heart by better recapitulating its in vivo microenvironment, including its molecular, cellular and extracellular components14,22. CS generation from stem cell-derived cells in the hanging drops allow their cultures in defined conditions (e.g., cell types and ratio, proper tissue formation). Co-cultures of iCMs together with HCFs and HCAECs within CSs define the molecular and cellular crosstalk that regulates the heart pathophysiology, including its contractile function and response to drugs at concentrations found in the patient's bloodstream14. Due to these unique features, CSs have been utilized to model cardiac fibrosis, a severe consequence of myocardial infarction and heart failure21. Our previous studies showed how the presence of both endothelial cells and fibroblasts is critical for the recapitulation of the vascular microenvironment in the human heart, allowing the optimal deposition of fibroblast-derived ECM proteins, such as laminin, fibronectin and collagen type IV, localized in proximity of a developing endothelial cell network14,21.
DOX is a well-known cardiotoxic drug that may develop heart failure in cancer patients even 17 years after their treatment30. Nevertheless, it remains a drug of choice for the treatment of leukemia and lymphoma in paediatric patients and breast cancer in women30. DOX treatment in CSs has then been used to model heart failure (HF) in vitro to study both the mechanisms regulating toxicity in cardiac myocytes, endothelial cells and fibroblasts14 and to model HF-induced cardiac fibrosis21. Cell viability was statistically reduced in DOX treated CSs within 24 h when exposed to the drug at the concentration found in the bloodstream of cancer patients (between 5 and 10 μM)14 (Figure 2). Previous studies in our laboratory also demonstrated the toxic effects of DOX on both cardiac endothelial cells and fibroblasts via endothelial nitric oxide synthase (eNOS) using both genetic and chemical inhibitors of this signaling pathway14. The use of genetic (NOS3 shRNA) and chemical (N5-(1-iminoethyl)-L-ornithine, dihydrochloride, or L-NIO) antagonists of the eNOS signaling pathway as a downstream target of DOX prevented its toxic effects in both cardiac endothelial cells and fibroblasts14.
Contractile activity within CSs has also been measured thanks to the electrical coupling of cardiac cells when exposed to field potential stimulation. We found that CSs cultured with control media (DOX 0 μM) contract spontaneously and homogenously at a beating rate that can be paced by field stimulation within 1 and 3 Hz, comparable with a healthy human heart. On the other hand, DOX-treated CSs do not follow the electrical stimulation as they cannot contract. Together with the measurements of cell viability and toxicity using calcein-AM and ethidium homodimer, this functional assay for CS contractile function allow the evaluation of the complex scenario typical of the human heart in vitro, currently not achievable with other models. Compared to contractile activity measurements of single cardiac cells using the same system, we are not able to visualize and measure the sarcomere in CSs. Therefore, we are limited to measurements of % spheroid shortening over time, an assay we had to develop within our laboratory. As we control the number of cells, we co-culture in each CS and therefore the size of each CS, we utilize CSs with similar size that indeed present homogenous contractile function. However, even in case we generated CSs of different sizes, their contractile activity did not change.
It is also important to report that the multicellular nature of CSs makes them heavy enough to localize at the bottom of the coverslip in the Ion-Optix system, even in case they are superfused. Based on the fact that CSs sit by themselves in a specific position, we do not need to make them adhere to the coverlip, on the contrary of what is commonly done with single cardiac cells in most laboratories.
The microscopic analysis of CSs stained with antibodies against cardiac troponin T, CD31/PECAM, and PECAM (as markers for iCMs, HCAECs, and HCFs, respectively) showed the formation of a endothelial cell network (Figure 1, blue). To fully exclude necrosis in the inner part of CSs, spatial evaluation of cell viability was performed in our laboratory by confocal analysis of calcein-AM/ethidium homodimer stained CSs (data not shown). However, it is important to acknowledge that future developments in the biofabrication field to better recapitulate other complex features typical of the human heart in vivo, currently not available in the existing model. These include: i) contractile function typical of adult cardiomyocytes; ii) blood flow and pressure forces; iii) paracrine signaling; iv) immune response, which will be critical to improve this and other in vitro cardiac models6. As any other model aims at recapitulating major features of either a healthy tissue or a disease state, the protocol for the generation and use of CS described in this manuscript aims at helping the researcher at addressing specific questions, that may not be exhaustive using this approach. For instance, the potential use of patient-derived cells for the generation of CSs would provide tools for personalized medicine, currently not available using commonly available high-throughput assays for cardiovascular research.
In conclusion, we demonstrated a simple way to better recapitulate the human heart microenvironment using cardiac cells. Cardiac spheroids present an endothelial cell network that better recapitulates the one present in the human heart compared to monolayer cultures of cardiac cells.Given their unique features, they represent advanced tools for in vitro testing for cardiovascular research. Future studies using patient-derived cells could provide options for personalized medicine and novel therapies to both prevent and better treat cardiovascular disease.
Disclosures
None
Acknowledgements
A special thanks to Nat Johnston for the recording and editing the video.
Poonam Sharma was supported by University of Newcastle with UNIPRS and UNRS Central & Faculty School (UNRSC5050) scholarships. Carmine Gentile was supported by a UTS Seed Funding, Catholic Archdiocese of Sydney Grant for Adult Stem Cell Research and a Sydney Medical School Foundation Cardiothoracic Surgery Research Grant.
Materials
| Name | Company | Catalog Number | Comments |
| Bovine Serum Albumin (BSA) | Sigma-Aldrich | A1933 | |
| Donkey anti-mouse Secondary Antibodies | Jackson Immunological Research Labs, Inc. | 715-165-150 | Cyanine Cy3-conjugated secondary antibody |
| Doxorubicin hydrochloride | Sigma-Aldrich | D1515 | |
| Fibronectin | Sigma-Aldrich | F1141-1MG | From Bovine Plasma |
| Human cardiac fibroblasts (HCFs) | Cell Applications, Inc., San Diego, CA, USA | 306AK-05a | 5x10^5 Cells (Adult), Medium & Subculture Reagents |
| Human coronary artery endothelial cells (HCAECs) | Cell Applications, Inc., San Diego, CA, USA | 300K-05a | 5x10^5 Cells (Adult), Medium & Subculture Reagents |
| Human iPSC-derived cardiomyocytes (iCMs) | Fujifilm Cellular Dynamics, Inc. | R1057 | iCell Cardiomyocytes Kit, 01434 |
| HCF Growth medium | Cell Applications, Inc., San Diego, CA, USA | 316-500 | |
| Human MesoEndo Cell Growth Medium | Cell Applications, Inc., San Diego, CA, USA | 212-500 | |
| LIVE/DEAD Viability/Cytotoxicity Kit | Invitrogen, Carlsbad, CA, USA | L3224 | |
| Maintenance Medium (iCells) | Fujifilm Cellular Dynamics, Inc. | R1057 | iCell Cardiomyocytes Kit, 01434 |
| Mouse Monoclonal anti-human CD31/PECAM | BD Pharmingen, San Diego, CA, USA | 566177 | |
| NucBlue Live ReadyProbe Reagent (Hoechst 33342) | Invitrogen, Carlsbad, CA, USA | R37605 | |
| Paraformaldehyde | Sigma-Aldrich | P6148 | |
| Phosphate-Buffered Saline | Sigma-Aldrich | D8537 | |
| Plating Medium (iCells) | Fujifilm Cellular Dynamics, Inc. | R1057 | iCell Cardiomyocytes Kit, 01434 |
| Rat Tail Collagen | Sigma-Aldrich | C3867 | |
| Sodium Azide | Sigma-Aldrich | S2002 | |
| Trypsin–EDTA, 0.25% | Gibco, Thermofisher Scientific | 25200072 | |
| Trypan Blue Solution, 0.4% | Gibco, Thermofisher Scientific | 15250061 | |
| Triton-X 100 | Sigma-Aldrich | X100 | |
| Tissue culture flasks (T25) | Thermofisher Scientific | 156367 | |
| 96-well Flat Clear Bottom Black Polystyrene TC-treated Microplates | Corning, New York, USA | 3603 | |
| 384-Well Hanging Drop Plate | 3D Biomatrix, Ann Arbor, MI, USA | HDP1385 |
References
- Dzobo, K., et al. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells International. 2018, 2495848 (2018).
- Pecha, S., Eschenhagen, T., Reichenspurner, H. Myocardial tissue engineering for cardiac repair. The Journal of Heart and Lung Transplantation. 35 (3), 294-298 (2016).
- Sekiya, S., Shimizu, T. Introduction of vasculature in engineered three-dimensional tissue. Inflammation and Regeneration. 37, 25 (2017).
- Rodrigues, I. C. P., Kaasi, A., Maciel Filho, R., Jardini, A. L., Gabriel, L. P. Cardiac tissue engineering: current state-of-the-art materials, cells and tissue formation. Einstein. 16 (3), 4538 (2018).
- Pena, B., et al. Injectable Hydrogels for Cardiac Tissue Engineering. Macromolecular Bioscience. 18 (6), 1800079 (2018).
- Roche, C. D., Brereton, R. J. L., Ashton, A. W., Jackson, C., Gentile, C. Current challenges in three-dimensional bioprinting heart tissues for cardiac surgery. European Journal of Cardio-Thoracic Surgery. , (2020).
- Fleischer, S., Feiner, R., Dvir, T. Cardiac tissue engineering: from matrix design to the engineering of bionic hearts. Regenerative Medicine. 12 (3), 275-284 (2017).
- Fleischer, S., Tavakol, D. N., Vunjak-Novakovic, G. From Arteries to Capillaries: Approaches to Engineering Human Vasculature. Advanced Functional Materials. (1910811), 23 (2020).
- O'Donnell, B. T., Ives, C. J., Mohiuddin, O. A., Bunnell, B. A. Beyond the Present Constraints That Prevent a Wide Spread of Tissue Engineering and Regenerative Medicine Approaches. Frontiers in Bioengineering and Biotechnology. 7, 95 (2019).
- Gentile, C. Filling the Gaps between the In Vivo and In Vitro Microenvironment: Engineering of Spheroids for Stem Cell Technology. Current Stem Cell Research & Therarpy. 11 (8), 652-665 (2016).
- Kim, J. J., Hou, L., Huang, N. F. Vascularization of three-dimensional engineered tissues for regenerative medicine applications. Acta Biomaterialia. 41, 17-26 (2016).
- Gentile, C., Muise-Helmericks, R. C., Drake, C. J. VEGF-mediated phosphorylation of eNOS regulates angioblast and embryonic endothelial cell proliferation. Developmental Biology. 373 (1), 163-175 (2013).
- Sweeney, M., Foldes, G. It Takes Two: Endothelial-Perivascular Cell Cross-Talk in Vascular Development and Disease. Frontiers in Cardiovascular Medicine. 5, 154 (2018).
- Polonchuk, L., et al. Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Scientific Reports. 7 (1), 7005 (2017).
- Zhang, J., Zhu, W., Radisic, M., Vunjak-Novakovic, G. Can We Engineer a Human Cardiac Patch for Therapy. Circulation Research. 123 (2), 244-265 (2018).
- Langhans, S. A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Frontiers in Pharmacology. 9, 6 (2018).
- Sarker, M. D., Naghieh, S., Sharma, N. K., Chen, X. 3D biofabrication of vascular networks for tissue regeneration: A report on recent advances. Journal of Pharmaceutical Analysis. 8 (5), 277-296 (2018).
- Zamani, M., Karaca, E., Huang, N. F. Multicellular Interactions in 3D Engineered Myocardial Tissue. Frontiers in Cardiovascular Medicine. 5, 147 (2018).
- Grounds, M. D. Obstacles and challenges for tissue engineering and regenerative medicine: Australian nuances. Clinical and Experimental Pharmacology and Physiology. 45 (4), 390-400 (2018).
- Campbell, M., Reis, R. L., et al. . Encyclopedia of Tissue Engineering and Regenerative Medicine. , 387-393 (2019).
- Figtree, G. A., Bubb, K. J., Tang, O., Kizana, E., Gentile, C. Vascularized Cardiac Spheroids as Novel 3D in vitro Models to Study Cardiac Fibrosis. Cells Tissues Organs. 204 (3-4), 191-198 (2017).
- Campbell, M., Chabria, M., Figtree, G. A., Polonchuk, L., Gentile, C. Stem Cell-Derived Cardiac Spheroids as 3D In Vitro Models of the Human Heart Microenvironment. Methods in Molecular Biology. 2002, 51-59 (2019).
- Pagliari, S., et al. A multistep procedure to prepare pre-vascularized cardiac tissue constructs using adult stem sells, dynamic cell cultures, and porous scaffolds. Frontiers in Physiology. 5, 210 (2014).
- Gentile, C., et al. VEGF-mediated fusion in the generation of uniluminal vascular spheroids. Developmental Dynamics. 237 (10), 2918-2925 (2008).
- Visconti, R. P., et al. Towards organ printing: engineering an intra-organ branched vascular tree. Expert Opinion on Biological Therapy. 10 (3), 409-420 (2010).
- Fleming, P. A., et al. Fusion of uniluminal vascular spheroids: a model for assembly of blood vessels. Developmental Dynamics. 239 (2), 398-406 (2010).
- Traore, M. A., George, S. C. Tissue Engineering the Vascular Tree. Tissue Engineering Part B: Reviews. 23 (6), 505-514 (2017).
- Sakaguchi, K., Shimizu, T., Okano, T. Construction of three-dimensional vascularized cardiac tissue with cell sheet engineering. Journal of Controlled Release. 205, 83-88 (2015).
- Chen, F. M., Liu, X. Advancing biomaterials of human origin for tissue engineering. Progress in Polymer Science. 53, 86-168 (2016).
- Kalyanaraman, B. Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: Have we been barking up the wrong tree. Redox Biology. 29, 101394 (2020).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved