A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Optrode Array for Simultaneous Optogenetic Modulation and Electrical Neural Recording
* These authors contributed equally
In This Article
Summary
Here, we present the fabrication method of an optrode system with optical fibers for light delivery and an electrode array for neural recording. In vivo experiments with transgenic mice expressing channelrhodopsin-2 show the feasibility of the system for simultaneous optogenetic stimulation and electrophysiological recording.
Abstract
During the last decade, optogenetics has become an essential tool for the investigation of neural signaling due to its unique capability of selective neural modulation or monitoring. As specific types of neuronal cells can be genetically modified to express opsin proteins, optogenetics enables optical stimulation or inhibition of the selected neurons. There have been several technological advances in the optical system for optogenetics. Recently, it was proposed to combine the optical waveguide for light delivery with electrophysiological recording to simultaneously monitor the neural responses to optogenetic stimulation or inhibition. In this study, an implantable optrode array (2x2 optical fibers) was developed with embedded multichannel electrodes.
A light-emitting diode (LED) was employed as a light source, and a microfabricated microlens array was integrated to provide sufficient light power at the tip of the optical fibers. The optrode array system comprises the disposable part and the reusable part. The disposable part has optical fibers and electrodes, while the reusable part has the LED and electronic circuitry for light control and neural signal processing. The novel design of the implantable optrode array system is introduced in the accompanying video in addition to the procedure of the optrode implantation surgery, optogenetic light stimulation, and the electrophysiological neural recording. The results of in vivo experiments successfully showed time-locked neural spikes evoked by the light stimuli from hippocampal excitatory neurons of mice.
Introduction
Recording and controlling neural activity is essential for understanding how the brain functions in a neural network and at cellular levels. Conventional electrophysiological recording methods include the patch clamp1,2,3,4 using a micropipette and extracellular recording using microneural electrodes5,6,7,8. As a neuromodulation method, electrical stimulation has been frequently used to directly stimulate a focal brain region through direct or indirect depolarization of neuronal cells. However, the electrical method cannot distinguish neuronal cell types for recording or stimulation because the electrical currents spread in all directions.
As an emerging technology, optogenetics has ushered in a new era in understanding how the nervous system works9,10,11,12,13,14,15,16. The essence of optogenetic techniques is to use light to control the activity of light-sensitive opsin proteins expressed by genetically modified cells. Thus, optogenetics enables the sophisticated modulation or monitoring of genetically selected cells in complicated neural circuits14,17. The wider use of the optogenetic approach has necessitated simultaneous neural recording to directly confirm optical neuromodulation. Therefore, an integrated device with light control and recording functions would be extremely valuable16,18,19,20,21,22,23,24,25.
There are limitations of conventional, laser-based optogenetic stimulation, which requires a bulky and expensive light delivery system26,27,28,29,30. Therefore, some research groups employed µLED-based silicon probes to minimize the size of the light delivery system31,32,33,34. However, there is a risk of thermal brain damage caused by direct contact with µLEDs due to the low energy conversion efficiency of LEDs. Light waveguides, such as optical fibers, SU-8, and silicon oxynitride (SiON), have been applied to avoid thermal damage30,35,36,37,38,39. However, this strategy also has a drawback due to its low coupling efficiency between light sources and the waveguides.
The microlens array was previously introduced to enhance the light coupling efficiency between LEDs and optical fibers40. An optrode system was developed based on microelectromechanical systems (MEMS) technologies for optical stimulation and electrical recording on a microscale40. The microlens array between an LED and optical fibers increased the light efficiency by 3.13 dB. As shown in Figure 1, a 2x2 optical fiber array is aligned on the 4x4 microlens array, and the LED is positioned below the microlens array. The 2x2 optical fibers are mounted instead of 4x4 to reduce brain damage. A tungsten electrode array is positioned adjacent to the optrode array using silicon via holes for electrophysiological recording (Figure 1B).
The system consists of a top disposable part and detachable bottom parts. The top disposable part, which includes the optical fiber array, microlens array, and the tungsten electrode array, is designed to be permanently implanted into the brain for in vivo experiments. The bottom part includes an LED light source and an external power supply line, which is easily removable and reusable for another animal experiment. An attachable plastic cover protects the disposable part when the detachable part is removed.
The feasibility of the system is verified by implantation into the brains of transgenic mice expressing channelrhodopsin-2 (ChR2) in Ca2+/calmodulin-dependent protein kinase II-positive neurons (CaMKIIα::ChR2 mouse). Recording electrodes were used to record the neural activities from individual neurons during optical stimulation of the neurons.
Protocol
The animal care and surgical procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the Ewha Womans University (no. 20-029).
1. Preparation of an optrode array (Figure 1 and Figure 2)
- Attach optical fibers with the microlens array.
- Remove the passivation coating of the optical fiber and cut it into 5 mm-long pieces using a precision optical fiber cleaver.
- Dip the optical fiber in the clear UV resin and place the optical fibers on the microlens array.
- Cure the resin using a UV lamp.
- Attach the microlens array to the 3D printed housing using epoxy.
- Align perfluoroalkoxy (PFA)-coated tungsten wires.
- Solder the 4 pieces of 30 mm-long PFA tungsten wires and 1 silver wire to the 5-pin, 1.27 mm pitch female connector. Use silver wire as a reference electrode.
- Connect the 1.27 mm pitch female connector to the headstage preamplifier.
- Carefully put tungsten wires through the silicon via the holes (Figure 1B) to help the fine alignment of the tungsten electrodes.
- Cut the aligned tungsten wires 1 mm shorter than the optical fiber length.
- Attach the connector to the 3D printed housing using epoxy.
- LED placement
- Place the LED in the 3D printed housing.
- Connect the LED to the driving circuit that generates pulse width modulation (PWM).
NOTE: The PWM pulse is generated by the microcontroller. - Given the noise induced by the LED driving circuit, ensure the recording electrodes are 50 mm apart from the LED driving circuit.
- Measure the light intensity at the end of the optical fiber tip using a photodiode.
- Immerse the tungsten electrodes and optical fibers in alcohol for 15 min. Then immerse the system in sterile D.I water and sterilize with ethylene oxide gas.
2. Implantation surgery (Figure 3 and Figure 4)
NOTE: Sterile technique must be followed during surgery.
- Prepare the transgenic animals, which are genetically modified to express light-sensitive opsin proteins in the specific type of neuronal cells.
NOTE: A male, 2 months old, transgenic mouse expressing channelrhodopsin-2 (ChR2) in Ca2+/calmodulin-dependent protein kinase II-positive neurons (CaMKIIα::ChR2 mouse) was used in this study41. - Anesthetize the mouse with a ketamine-xylazine cocktail-a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg)-by intraperitoneal administration.
- Check the anesthetized animal every 30 min by monitoring whisker movement and reaction to paw pinch.
- Inject the ketamine-xylazine cocktail at half of the initial dose if additional anesthesia is needed.
- Shave the head skin and place the anesthetized mouse in the stereotactic frame.
NOTE: Perform surgical skin prep and drape animal. Skin preparation involves hair removal followed by cleaning of the skin with antimicrobial agents.- Position the head within the stereotactic frame and insert ear bars into the meatus.
- Center the mouse head in the stereotactic frame by repeatedly loosening and tightening the ear bars for exact positioning.
- Place the incisor bar so that the upper incisors hook over the front inside edge.
- Adjust the incisor bar for setting the height of the incisor bar, and tighten the nose clamp against the snout.
- Turn on the thermal heating pad embedded in the stereotactic frame in advance to maintain the body temperature at 37 °C throughout the surgical procedures.
- Put the thermal probe into the mouse's rectum for homeothermic regulation.
NOTE: Ear bar placement must be checked by gently grasping and swaying the snout. The ear bar must be readjusted if the snout can be moved more than ~4 mm laterally.
- Cover the eyes with petroleum jelly (see the Table of Materials) to prevent dryness.
- Inject 1% lidocaine into the scalp. Lift the skin of the head with forceps, secure an injection space, and inject the lidocaine under the scalp.
- Perform a sagittal incision using a scalpel and fine scissors. Hold the incised skin with a microclamp to broaden the visibility of the surgical area.
NOTE: The incision length is <1 cm above the bregma and caudal edge of the interparietal bone. - Remove the periosteum using cotton swabs. If there is bleeding, cauterize the skull using a bovie to seal the blood vessels.
- Clean the skull with saline and mark the craniotomy sites using a manipulator arm: hippocampus Anterior-Posterior (AP) −1.8 to −2.8 mm, Medial-Lateral (ML) 0.5-2.5 mm, and Dorsal-Ventral (DV) −1 to −2 mm.
NOTE: Considering the thickness of the mouse skull, insert the optrode array -1.2 to -2.2 mm from the exposed brain area. Given the hippocampal pathway, pyramidal cells of CA3 send their axons to CA1. Therefore, optical fibers are 1 mm longer than recording electrodes, so that the optical fibers are placed in CA3 and the recording electrodes in CA1. - Drill a hole above the cerebellum and insert a screw for ground. Put the ground screw 0.5 mm deep with a precision screw until it reaches the top of the cerebellum.
NOTE: Ensure tight insertion of the screw, which will be used as a ground electrode and a supporting structure to maximize the long-term stability of implantation, as it increases the three-dimensional surface for adherence of the dental cement. - Drill the marked area and remove a piece of the skull with forceps.
- Bend a 26 G needle tip to clockwise 120°, and expose the brain area by inserting the bevel side of the needle faced upward. Be careful not to damage the brain and blood vessels.
- Clean the exposed brain with saline to wash out bone dust and extraneous matter.
- Fix the optrode array in the manipulator arm and move close to the exposed area.
NOTE: When placing the device, recalculating the target area again from the bregma can increase the accuracy of the placement. - Slowly insert the optrode array and connect the ground screw to the silver wire attached to the system42.
NOTE: In this process, the array must be tightly fixed to the manipulator arm to minimize wobbling to prevent brain damage from micromotion during insertion. Insertion must be performed slowly, with rest for 10 min to account for swelling of the brain that may have been pressed after insertion. Insertion speed under 1 µm/s into the brain tissue is recommended for the high quality of the signal-to-noise ratio and the number of separable single units42. - If there is bleeding, apply direct pressure to bleeders with a dry cotton swab or use cautery. Do not move on to the next steps until bleeding is completely stopped.
- Insert gel foam between the exposed brain and the device to protect chemicals from direct contact with the brain.
NOTE: Gel foam also helps hemostasis and keeps the brain moist. - Wipe the skull with cotton swabs to remove moisture as much as possible, and proceed to the next fixation step.
- Carefully apply dental cement to the skull to fix the device and cover the gel foam. Before the dental cement is completely hardened, separate the scalp if attached to dental cement. Pull the incised skin with forceps to cover the hardened dental cement and suture the scalp. Then, release the device from the manipulator arm.
NOTE: Ensure that the dental cement does not stick to the skin and only covers the skull region. When the skin grows, it can push the cement away and, eventually, the device will fall off. This will cause a critical problem for long-term in vivo experiments.
3. Recovery and implant care
- Take the mouse out of the stereotactic frame.
- Administer Carprofen solution using a 26 G needle. Inject once before surgery and 12 h (next morning in case of afternoon surgery) and 24 h after surgery to reduce pain.
NOTE: The drug concentration in the bottle is 50 mg/mL, and it is stored at 4 °C. Carprofen is viscous and needs to be diluted in sterile water 1:10. If sterility is maintained, the solution can be stored for up to 4 weeks. To maintain the effective duration of the drug, inject the Carprofen solution at 12 h intervals. - Place the mouse on the heating pad and check if it recovers well from the anesthesia.
NOTE: The animal is not left unattended until it regains sufficient consciousness. - Remove the suture threads 7 - 10 days after surgery.
- Allow the animal to recover for 1 week. Monitor food and water intake during recovery time. Administer the analgesic and check for the signs of discomfort or pain.
NOTE: The animals that have undergone surgery cannot stay with the other animals until they fully recover.- During the recovery period, weigh the mouse every day to check for weight changes. Sacrifice the mouse (see section 6) if there is 20% weight loss compared to age and sex matched control animals.
4. Optogenetic stimulation and electrophysiological recording
- Anesthetize the mouse and place the anesthetized mouse in the stereotactic frame.
- Set up the stimulation parameters.
- Set the light pulse recipe to 4% duty cycle and 10 Hz frequency43. Set up the light stimulation for 2 s (20 pulses) during neural recording.
- Use a 50 mA current to drive 3 mW/mm2 light intensity at the optical fiber tip. Measure the light power using a photodiode and power meter and divide the light power by the optical fiber facet area.
- Take off the plastic cover and attach the upper part containing the light delivery system with the reusable LED and circuits.
- Connect the headstage preamplifier to the implanted 5 pin connector.
- Open the software to record neural signals.
- Set up the software filters. Use a bandpass filter at 0.1-20 kHz and a notch filter at 60 Hz. Set the amplifier sampling rate to 20 kHz.
- Before the recording, check if the neural spike signals are detected.
NOTE: Noise cancellation is important because neural signals are too small. If the noise level is too high, neural signals may be obscured by noise and become invisible. Use appropriate grounding and electromagnetic wave shielding to reduce noise. - After the signal check, record neural signals without light stimulation for baseline recording.
- Deliver LED light and simultaneously record neuronal responses (Figure 5 and Figure 6).
NOTE: Once stimulated, the next stimulation experiment must be performed after an interval of at least 5 min so that the neuronal activity evoked by the previous stimulation does not affect the next experiment.
5. Data analysis
- Load the acquired data using MATLAB program.
- Obtain single neural activities using a spike sorting algorithm "Wave-Clus"44,45. Detect the spikes in each channel by an amplitude threshold as in Eq (1)45.
Threshold = 5 × median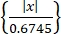 (1)
(1)
Where x is the bandpass-filtered signal.- To install "Wave-Clus," refer to the download link in the Table of Materials.
- To open the graphical user interface (GUI), type wave_clus in the command prompt of MATLAB.
- Type Get_spikes('filename.ext') function for file extension prepared to spike detection. Then, run Do_clustering('filename_spikes.mat') for spike sorting.
- Count the sorted spikes before, during, and after light stimulation with specific time bins. Use 0.2 s and 2 s time bins for analysis.
- Plot the number of spikes from each recording channel.
NOTE: Mean with standard error of the means (SEM) of the data from the results of 8 repeated experiments was calculated and plotted.
6. Euthanasia
- After all the experiments, sacrifice the mouse by carbon dioxide (CO2) inhalation.
- Without precharging the chamber, place the mouse in the transparent euthanasia chamber, without CO2 supply and/or leaks.
- Turn on the CO2 gas, and displace the air at a rate of 30 - 50% of the volume of euthanasia chamber air per min.
NOTE: A flowmeter must be connected to the CO2 gas cylinder to ensure that the air displacement rate is 30-50% of the volume of the euthanasia chamber per min. - Monitor the sacrificed mouse for lack of respiration and changed eye color.
NOTE: The expected time for euthanasia is usually within 10 to 15 min. - After observing the death signs, remove the mouse from the euthanasia chamber.
- To complete the euthanasia procedure, verify death by confirming respiratory and cardiac arrest.
Results
The optrode system is successfully fabricated to provide sufficient light power to activate the target neurons. The fine alignment of the tungsten electrodes is achieved through the microfabricated silicon via the holes. The measured light intensity is 3.6 mW/mm2 at the optical fiber tip when 50 mA current is applied. The microlens increased the light efficiency by 3.13 dB. Due to the microlens array, which enhances the light coupling, the applied current is approximately half of the current required to achiev...
Discussion
The feasibility of the system for simultaneous optogenetic stimulation and electrophysiological recording was verified (Figure 6). The big spikes during light stimulation are photoelectric artifacts occurring at the same time as the light stimulation (Figure 6A). This is clear in the zoomed view of the waveform in the red dashed rectangle (Figure 6A). As shown in Figure 6A, the photoelectrical artifacts...
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This research was supported by Convergent Technology R&D Program for Human Augmentation through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (NRF-2019M3C1B8090805), and supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no. 2019R1A2C1088909). We thank Seung-Hee Lee's laboratory at the Department of Biological Sciences, KAIST, Daejeon, Korea, for kindly providing the transgenic mice.
Materials
| Name | Company | Catalog Number | Comments |
| 5-pin Connector | NW3 | HD127K | 1.27 mm (.050") pitch |
| Bovie | Fine Science Tools(F.S.T) | 18010-00 | High Temperature Cautery Kit |
| Data Acquisition Software | Intan Technologies, LLC | USB Interface Board software | Work with the RHD USB Interface Board |
| Dental Cement | Lang Dental Manufacturing Company, Inc. | 1223CLR | Use Jet Liquid and powder in jet denture repair package |
| Digital Manipulator Arm | Stoelting Co. | 51904/51906 | Left, Right each Digital Manipulator Arm, 3-Axes, Add-On |
| Gel Foam | Cutanplast | Standard (70*50*10 mm) | Sterile re-absorbable gelatin sponge with a haemostatic effect |
| Headstage Preamplifier | Intan Technologies, LLC | #C3314 | RHD 16-Channel Recording Headstages |
| Heating Pad | Stoelting Co. | 53800R | Stoelting Rodent Warmer X1 with Rat Heating Pad |
| LED | OSLON | GB CS8PM1.13 | λ typ. 470 nm, Viewing angle 80 °, Forward voltage 2.85 V |
| MATLAB | MathWorks, Inc. | R2019a | |
| Micro Clamp | SURGIWAY | 12-1002-04 | Straight type, Serre-fine DIEFFENBACH droite 3.5 cm |
| Optical Fiber | Thorlabs, Inc. | FT200UMT | 0.39 NA, Ø 200 µm Core Multimode Optical Fiber, High OH for 300 - 1200 nm |
| PFA-Coated Tungsten Wire | A-M System | Custom ordered | Rod type, Ø 101.6 μm (.004") |
| Photodiode | Thorlabs | S121C | |
| power meter | Thorlabs Inc. | PM100D | |
| Precision cleaver | FITEL | S326 | Fiber slicer tool |
| Prism | GraphPad | 5.01 version | |
| Scalpel | Feather™ | #20 | Scalpel blade with 100mm long Scalpel Handle |
| screw | Nasa Korea | stainless steel | diameter: 1.2 mm, length: 3 mm |
| Silver Wire | The Nilaco Corporation | AG-401265 | Ø 200 µm |
| Stereotaxic Fxrame | Stoelting Co. | 51500D | Digital new standard stereotaxic, rat and mouse |
| suture | ETHICON | W9106 | suture size: 4-0, length:75 cm, wire diameter: 4-0 |
| Vaseline | Unilever PLC | Original | 100% pure petroleum jelly |
| Wave_Clus | N/A | N/A | https://github.com/csn-le/wave_clus |
References
- Wang, Y., Liu, Y. Z., Wang, S. Y., Wang, Z. In vivo whole-cell recording with high success rate in anaesthetized and awake mammalian brains. Molecular Brain. 9 (1), 86 (2016).
- Segev, A., Garcia-Oscos, F., Kourrich, S. Whole-cell patch-clamp recordings in brain slices. Journal of Visualized Experiments: JoVE. (112), e54024 (2016).
- Lee, D., Shtengel, G., Osborne, J. E., Lee, A. K. Anesthetized- and awake-patched whole-cell recordings in freely moving rats using UV-cured collar-based electrode stabilization. Nature Protocols. 9 (12), 2784-2795 (2014).
- Tao, C., Zhang, G., Xiong, Y., Zhou, Y. Functional dissection of synaptic circuits: in vivo patch-clamp recording in neuroscience. Frontiers in Neural Circuits. 9, 23 (2015).
- Henze, D. A., et al. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. Journal of Neurophysiology. 84 (1), 390-400 (2000).
- Takahashi, S., Anzai, Y., Sakurai, Y. Automatic sorting for multi-neuronal activity recorded with tetrodes in the presence of overlapping spikes. Journal of Neurophysiology. 89 (4), 2245-2258 (2003).
- Buzsáki, G. Large-scale recording of neuronal ensembles. Nature Neuroscience. 7 (5), 446-451 (2004).
- Rossant, C., et al. Spike sorting for large, dense electrode arrays. Nature Neuroscience. 19 (4), 634-641 (2016).
- Balasubramaniam, S., et al. Wireless communications for optogenetics-based brain stimulation: present technology and future challenges. IEEE Communications Magazine. 56 (7), 218-224 (2018).
- Bedbrook, C. N., et al. Machine learning-guided channelrhodopsin engineering enables minimally invasive optogenetics. Nature Methods. 16 (11), 1176-1184 (2019).
- Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G., Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neuroscience. 8 (9), 1263-1268 (2005).
- Deng, W., Goldys, E. M., Farnham, M. M., Pilowsky, P. M. Optogenetics, the intersection between physics and neuroscience: light stimulation of neurons in physiological conditions. American journal of physiology. Regulatory, Integrative and Comparative Physiology. 307 (11), 1292-1302 (2014).
- Fenno, L., Yizhar, O., Deisseroth, K. The development and application of optogenetics. Annual Review Neuroscience. 34, 389-412 (2011).
- Mahmoudi, P., Veladi, H., Pakdel, F. G. Optogenetics, tools and applications in neurobiology. Journal of Medical Signals and Sensors. 7 (2), 71-79 (2017).
- Sasaki, Y., et al. Near-infrared optogenetic genome engineering based on photon-upconversion hydrogels. Angewandte Chemie International Edition in English. 58 (49), 17827-17833 (2019).
- Zhang, Y., et al. Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics. Proceedings of National Academy of Sciences of the United States of America. 116 (43), 21427-21437 (2019).
- Bernstein, J. G., Boyden, E. S. Optogenetic tools for analyzing the neural circuits of behavior. Trends in Cognitive Sciences. 15 (12), 592-600 (2011).
- Wang, J., et al. Integrated device for combined optical neuromodulation and electrical recording for chronic in vivo applications. Journal of Neural Engineering. 9 (1), 016001 (2012).
- Royer, S., et al. Multi-array silicon probes with integrated optical fibers: light-assisted perturbation and recording of local neural circuits in the behaving animal. European Journal of Neuroscience. 31 (12), 2279-2291 (2010).
- Zhang, J., et al. Integrated device for optical stimulation and spatiotemporal electrical recording of neural activity in light-sensitized brain tissue. Journal of Neural Engineering. 6 (5), 055007 (2009).
- Park, S. I., et al. Stretchable multichannel antennas in soft wireless optoelectronic implants for optogenetics. Proceedings of National Academy of Sciences of the United States of America. 113 (50), 8169-8177 (2016).
- Kravitz, A. V., Owen, S. F., Kreitzer, A. C. Optogenetic identification of striatal projection neuron subtypes during in vivo recordings. Brain Research. 1511, 21-32 (2013).
- Aravanis, A. M., et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. Journal of Neural Engineering. 4 (3), 143-156 (2007).
- Anikeeva, P., et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nature Neuroscience. 15 (1), 163-170 (2011).
- Obaid, S. N., et al. Multifunctional flexible biointerfaces for simultaneous colocalized optophysiology and electrophysiology. Advanced Functional Materials. 30 (24), 1910027 (2020).
- Wang, L., et al. An artefact-resist optrode with internal shielding structure for low-noise neural modulation. Journal of Neural Engineering. 17 (4), 046024 (2020).
- Shin, H., et al. Multifunctional multi-shank neural probe for investigating and modulating long-range neural circuits in vivo. Nature Communications. 10 (1), 3777 (2019).
- Kampasi, K., et al. Dual color optogenetic control of neural populations using low-noise, multishank optoelectrodes. Microsystem & Nanoengineering. 4, 10 (2018).
- Schwaerzle, M., Paul, O., Ruther, P. Compact silicon-based optrode with integrated laser diode chips, SU-8 waveguides and platinum electrodes for optogenetic applications. Journal of Micromechanics and Microengineering. 27 (6), 065004 (2017).
- Son, Y., et al. In vivo optical modulation of neural signals using monolithically integrated two-dimensional neural probe arrays. Scientific Reports. 5, 15466 (2015).
- Yasunaga, H., et al. Development of a neural probe integrated with high-efficiency MicroLEDs for in vivo application. Japanese Journal of Applied Physics. 60 (1), 016503 (2020).
- Kim, K., et al. Artifact-free and high-temporal-resolution in vivo opto-electrophysiology with microLED optoelectrodes. Nature Communications. 11 (1), 2063 (2020).
- Mendrela, A. E., et al. A high-resolution opto-electrophysiology system with a miniature integrated headstage. IEEE Transactions on Biomedical Circuits and Systems. 12 (5), 1065-1075 (2018).
- Scharf, R., et al. Depth-specific optogenetic control in vivo with a scalable, high-density muLED neural probe. Scientific Reports. 6, 28381 (2016).
- Oh, K., Sonsi, Y. -. A., Ha, S. Optogenetic stimulator with µLED-coupled optical fiber on flexile substrate via 3D printed mount. 2021 21st International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers). , 1476-1479 (2021).
- McAlinden, N., et al. Multisite microLED optrode array for neural interfacing. Neurophotonics. 6 (3), 035010 (2019).
- Kwon, K. Y., Lee, H. M., Ghovanloo, M., Weber, A., Li, W. Design, fabrication, and packaging of an integrated, wirelessly-powered optrode array for optogenetics application. Frontiers in Systems Neuroscience. 9, 69 (2015).
- Bernstein, J. G., Allen, B. D., Guerra, A. A., Boyden, E. S. Processes for design, construction and utilisation of arrays of light-emitting diodes and light-emitting diode-coupled optical fibres for multi-site brain light delivery. Journal of Engineering. , (2015).
- Stark, E., Koos, T., Buzsaki, G. Diode probes for spatiotemporal optical control of multiple neurons in freely moving animals. Journal of Neurophysiology. 108 (1), 349-363 (2012).
- Jeon, S., et al. Implantable optrode array for optogenetic modulation and electrical neural recording. Micromachines. 12 (6), 725 (2021).
- Song, Y. H., et al. A neural circuit for auditory dominance over visual perception. Neuron. 93 (4), 940-954 (2017).
- Fiáth, R., et al. Slow insertion of silicon probes improves the quality of acute neuronal recordings. Scientific Reports. 9 (1), 111 (2019).
- Melchior, J. R., Ferris, M. J., Stuber, G. D., Riddle, D. R., Jones, S. R. Optogenetic versus electrical stimulation of dopamine terminals in the nucleus accumbens reveals local modulation of presynaptic release. Journal of Neurochemistry. 134 (5), 833-844 (2015).
- Quiroga, R. Q., Nadasdy, Z., Ben-Shaul, Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Computation. 16 (8), 1661-1687 (2004).
- Chaure, F. J., Rey, H. G., Quian Quiroga, R. A novel and fully automatic spike-sorting implementation with variable number of features. Journal of Neurophysiology. 120 (4), 1859-1871 (2018).
- Casanova, F., Carney, P. R., Sarntinoranont, M. Effect of needle insertion speed on tissue injury, stress, and backflow distribution for convection-enhanced delivery in the rat brain. PLoS One. 9 (4), 94919 (2014).
- Iseri, E., Kuzum, D. Implantable optoelectronic probes for in vivo optogenetics. Journal of Neural Engineering. 14 (3), 031001 (2017).
- Arias-Gil, G., Ohl, F. W., Takagaki, K., Lippert, M. T. Measurement, modeling, and prediction of temperature rise due to optogenetic brain stimulation. Neurophotonics. 3 (4), 045007 (2016).
- Jeon, S., et al. Multi-wavelength light emitting diode-based disposable optrode array for in vivo optogenetic modulation. Journal of Biophotonics. 12 (5), 201800343 (2019).
- Korposh, S., James, S. W., Lee, S. -. W., Tatam, R. P. Tapered optical fibre sensors: current trends and future perspectives. Sensors. 19 (10), 2294 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved