Normothermic Ex Vivo Pancreas Perfusion for the Preservation of Pancreas Allografts before Transplantation
In This Article
Summary
Normothermic ex vivo machine perfusion (NEVP) has scarcely been explored for the preservation of pancreas allografts. We present an innovative preservation technique for pancreas allografts before transplantation.
Abstract
Pancreas transplantation (PTx) is a curative treatment for people who live with the burden of a diagnosis of diabetes mellitus (DM). However, due to organ shortages and increasing numbers of patients being listed for PTx, new strategies are needed to increase the number of available grafts for transplantation.
Static cold storage (SCS) is considered the gold standard for standard criteria organs. However, standard criteria donors (SCD) are becoming scarce and new strategies that can increase the rate of organ acceptance from extended criteria donors (ECD) are urgently needed.
Normothermic ex vivo perfusion (NEVP) is one of the strategies that has become increasingly popular over the past couple of decades. This preservation method has already been used successfully in other organs (liver, kidneys, and lungs) but has been minimally explored in pancreas transplantation. The few papers that describe the method for pancreas show little success, edema being one of the major issues. The following manuscript describes the successful NEVP method and setup developed by our group to perfuse swine pancreas.
Introduction
According to the National Diabetes Statistics Report, a total of 28.7 million people in the United States were living with a diagnosis of diabetes in 2019. Approximately 1.8 million of these had a diagnosis of type 1 diabetes1. PTx is currently the most efficacious and only curative treatment for complicated type 1 diabetes mellitus2, and is a procedure that both increases life expectancy and quality of life of these patients3.
The pancreas is the most often discarded organ after retrieval from deceased donors4. With ongoing organ shortages and the increasing waiting list times, transplant centers are using more pancreas grafts from ECDs, including donation after circulatory death (DCD)5. Strategies to safely preserve, perfuse, assess, and repair allografts coming from extended criteria donors are needed.
NEVP has proven to be successful in the preservation of lung6, liver7, 8, and kidney grafts9,10. However, the number of groups working on machine perfusion for the pancreas, both hypothermic or normothermic, and the number of publications, are few and limited due to graft edema and injury11,12,13,14.
The objective of this study is to present a protocol for normothermic ex vivo pancreas perfusion (NEVPP), using a porcine model with the goal of eventually providing a platform for prolonged preservation, organ assessment, and repair before transplantation. This will allow other research groups to establish a perfusion model for the study of pancreas allografts.
Protocol
All animals used for this study received humane care in accordance with the ''Principles of Laboratory Animal Care'' formulated by the National Society for Medical Research and the ''Guide for the Care of Laboratory Animals'' published by the National Institutes of Health, Ontario, Canada. All studies were approved by the Animal Care Committee of the Toronto General Research Institute.
NOTE: This study protocol is based on a porcine model. The graft is stored in the cold for 2 h and then undergoes normothermic machine perfusion for 3 h prior to transplantation (Figure 1).
1. Animals
- Use male Yorkshire pigs (40-50 kg).
2. Organ procurement
NOTE: The preoperative procedure and part of the surgical procedure are the same as previous papers published by our group15 and is as follows:
- House the pigs in the research facility for a minimum of 7 days to allow for acclimation and to reduce their stress level.
- Fast the pigs for a minimum of 6 h before induction of the anesthesia.
- Sedate the pig with an intramuscular (IM) injection of midazolam (0.15 mg/kg), ketamine (25 mg/kg), and atropine (0.04 mg/kg).
NOTE: This is done in the housing facility. - Transfer the animal from the housing facility to the operating room (OR), where recovery of the organ will be performed.
- Position the pig in a supine position on the OR table and place a face mask with 2 L of oxygen and 5% of isoflurane until the jaw is relaxed.
- Visualize the vocal cords using laryngoscope and spray them with 2% lidocaine to prevent spasm during intubation. Replace the mask with oxygen and isoflurane for at least 30 s before attempting intubation.
- Introduce a 7 mm endotracheal tube and block the cuff with 5 mL of air. Use capnometry to ensure that the tube is in the correct position.
- Decrease the isoflurane gas to 2.5%. Turn on the ventilator and set it to 15-20 breaths/min and the tidal volume to 10-15 mL/kg bodyweight. Monitor heart rate and oxygen saturation constantly.
- Using the Seldinger technique16, introduce an 8.5 Fr. x 10 cm catheter into the jugular vein (either right or left).
- Use the jugular vein catheter to start an infusion of fentanyl (2.5 mL in 500 mL of Ringer) at 250 mL/ hr.
- Check muscular reflexes to determine the depth of anesthesia. Jaw tone is the most reliable muscular reflex 17.
NOTE: If the rigidity of mandibular muscles is noted, increase isoflurane and/or fentanyl infusion.
3. Surgical procedure
- Disinfect and cover the surgical field. Perform a midline incision from the xyphoid to the pubic symphysis. Extend the surgical field with a left lateral incision for better exposure.
- Dissect the inferior vena cava (IVC) from the abdominal aorta. Further free the aorta from the surrounding tissue and ligate the small lumbar aortic branches. Identify and place ligatures around both renal arteries.
NOTE: The ligatures must not be tied at this timepoint. - Once the back of the aorta is free, pass two ligatures around it. The lower ligature will eventually be tied just above the iliac artery bifurcation and upper ligature will be tied 5 cm above the previous tie.
- Dissect the hepatic hilum. Tie all arteries as close to the liver as possible. Identify the common bile duct, place two ligatures close to the liver, and divide the structure.
- Dissect around aorta but do not cut at this timepoint. Identify and dissect around the suprahepatic portion of aorta and place a tie around it.
NOTE: The ligatures must not be tied at this timepoint. - Open the lesser sac to allow ice to cool pancreas. Mobilize the pancreas as little as possible before flush.
- Administer 500 IU of heparin per kg of donor weight through the central line. Wait 5 min and start blood collection in citrate, phosphate, dextrose, saline, adenine, glucose, and mannitol (CPD/SAG-M) bags using the jugular catheter.
- Tie the inferior aortic ligature, cannulate the aorta with a flush line above the iliac bifurcation tie, and secure the cannula with an upper tie. Ligate both renal arteries.
- Tie the suprahepatic aorta (crossclamp) once enough blood has been collected (600 mL). Administer 10 mL of potassium chloride for sacrifice.
- Initiate a flush with University of Wisconsin (UW) preservation solution. Cut an opening in the portal vein (as high as possible) and cava for venting. Place ice in the abdominal cavity.
- Assess the pancreas tail and duodenum C-loop after flushing 1 L of UW solution. If adequate flush, begin dissection, identify, and clamp mesenteric vessels. Slow down flushing for the second liter of UW.
- Retrieve the pancreatic graft and a segment of cava or iliac vein for extension of portal vein.
NOTE: The pancreatic graft is removed with the spleen. - Place the organ inside an organ bag that is placed inside a basin filled with ice.
4. Back table preparation of the pancreatic graft (Figure 2A)
- Remove the flush line from distal part of the aorta and close with a tie. Fill the organ bag with the remaining UW solution. Free the pancreas from adherent tissue, including the spleen.
- Perform portal vein extension using previously recovered cava or iliac vein with 6-0 Prolene. Cannulate the portal vein and proximal aorta with a ¼ in x 3/8 in reducer.
- Cannulate the distal part of duodenum with Malecot catheter and tie. Clamp the end of catheter to avoid spilling of duodenal content. Oversaw mesenteric vessels with 4-0 Prolene.
- Register the weight of the graft. Keep the graft in static cold storage (SCS) until the start of the NEVPP.
5. Normothermic ex vivo pancreas perfusion (NEVPP)
NOTE: The perfusion circuit is made of neonatal cardiopulmonary bypass equipment (Figure 3).
- Attach the corresponding tubing to the oxygenator and to the venous reservoir, as well as the arterial line to the outflow of the oxygenator and place the bubble filter in its holder. Connect the purge line that goes from bubble filter to the venous reservoir. Open the bubble filter cap to let all the air out.
- Connect the venous line to the inlet of the venous reservoir. Connect the dialysis filter and tubing where dialysate will be infused. Connect the flow meter sensor, pressure lines, and the temperature probe. Connect the arterial and venous sample lines to the sample ports.
- Place the pancreas chamber (Figure 3) on a Mayo table and introduce the arterial and venous tubing through the holes intended for this purpose. Connect and turn on external heater unit.
- Place the suction tubing inside the roller pump and connect one end into the tubing that comes out from the chamber to collect the fluids, and the other end to the venous reservoir to collect all organ loses of perfusate.
- Connect the oxygen tubing (green) to the gas tank containing the carbogen mixture (95% O2/5% CO2) and the oxygenator. Connect the heater pump unit tubing to the oxygenator.
- Clamp arterial and venous outflow lines, as well as the outflow of the venous reservoir.
6. Preparation of the perfusate and priming of the circuit
- Fill the venous reservoir with the perfusate (Table 1).
- Use one syringe pump for continuous administration of the vasodilator (epoprostenol) at 8 mL/h into the arterial line. Use a second syringe pump for continuous administration of the enzyme inhibitor directly into the venous reservoir (15 mg, 10 mL/h).
- Turn on the heart lung machine (HLM) and start up the pressure, temperature, and timer panels. Turn on heater pump to warm the perfusion solution to 38 °C. Open the O2/CO2 supply.
- Remove the tubing clamp placed on the outflow of the venous reservoir, start the centrifugal pump, and take it up to 1,500 rpm. Clamp the tubing, bypassing the arterial filter and release air from the arterial filter. Zero the arterial and venous pressure lines.
7. Pancreas graft perfusion (Figure 2B)
- Open the organ bag where the pancreas is stored. Flush with 200 mL of albumin through the arterial cannula. Remove the pancreas from the ice and position inside the organ chamber. Confirm that the arterial and the venous tubing are air-free.
- Release clamp from the arterial side and clamp the shortcut between the arterial and venous tubing. Once blood starts coming out of arterial tubing, connect line to arterial cannula. Set arterial pressure to 20-25 mmHg, by regulating the speed of the centrifugal pump. Connect venous tubing once blood starts coming out from the venous cannula.
- Administer one vial of verapamil (2.5 mg/mL) directly on the arterial side, when the pancreas is completely connected and no major bleeds are observed.
- Record pressures, arterial flow, temperature, and duodenum secretion continuously. Collect blood, record duodenal output every hour, and assess hourly macroscopically for edema. Record the perfusion parameters and take samples for analysis (venous and arterial blood gas samples, as well as samples for amylase, lipase, and LDH).
- Disconnect arterial and venous tubing when perfusion is over, remove the graft from organ chamber, and flush with cold UW and weigh. Store graft on ice in a sterile organ bag until the moment of transplantation.
Representative Results
The upcoming data shows the representative results of seven experiments using a model of heart-beating donor pancreas retrieval. After cannulation of the aorta, flush with UW solution, and retrieval of the pancreas, the grafts were kept on SCS for 2 h while the red blood cells were prepared. NEVPP was performed in this model for 3 h, what we considered the least amount of time necessary for perfusion if graft assessment and repair are intended in the future. Samples and measurements were recorded at hourly timepoints. (0 = baseline, right after organ is connected to the circuit, 1 = 1 h, 2 = 2 h, 3 = 3 h).
Pancreas grafts were placed on an organ chamber that was custom designed for this purpose and includes a heater (Supplemental File). The purpose of NEVPP is to provide a near physiological environment for the organ. For this purpose, arterial pressure was set to remain between 20-25 mmHg in all perfusions. Pressure and flow were measured throughout the whole perfusion and remained stable (Figure 4). Metabolic activity was estimated by calculating the oxygen consumption of the graft using the following formula: [(pO2art-pO2ven) * flow / weight] (Figure 5). Measurements of pH, sodium, calcium, and HCO3 were within physiological values during the whole perfusion (Figure 6). Lactate and potassium levels decreased during the perfusion, and achieved close to normal values at 3 h (Figure 7). Since the circuit is a closed system, amylase and lipase levels are expected to increase during the perfusion (Figure 8). However, the increase in levels does not seem to correlate with the damage to the graft (Figure 9). A semiquantitative scale was used to score fat and parenchyma necrosis as well as islet cell integrity. (0 - no changes, 1 - mild changes, 2 - moderate changes, 3 - severe changes). This was done by a pathologist blinded to the experimental groups, and no signs of pancreatitis were observed.
Pancreas allografts were weighed before and after perfusion to assess edema (Table 2).
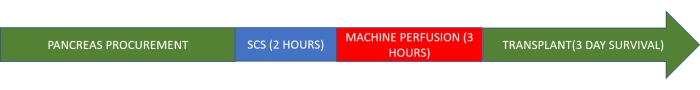
Figure 1. Study protocol. Please click here to view a larger version of this figure.

Figure 2. Pancreas before and after perfusion. (A) Before perfusion. (B) After perfusion. Please click here to view a larger version of this figure.
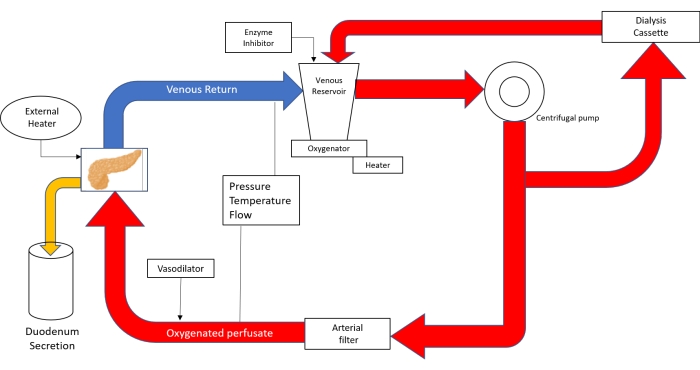
Figure 3. Schematic drawing of the perfusion circuit. With the use of Neonatal cardiopulmonary bypass technology; the perfusate is poured into the venous reservoir and then propelled with help of a centrifugal pump into the oxygenator. After leaving the oxygenator, the circuit divides into tubing that sends perfusate to the dialysis cassette and back to the reservoir and tubing that goes to the arterial filter. After passing the arterial bubble filter, the perfusate is driven with a pressure of 20-25 mmHg through the aorta into the pancreas. The venous outflow leads the perfusate back into the venous reservoir. (Adapted from 18). Please click here to view a larger version of this figure.
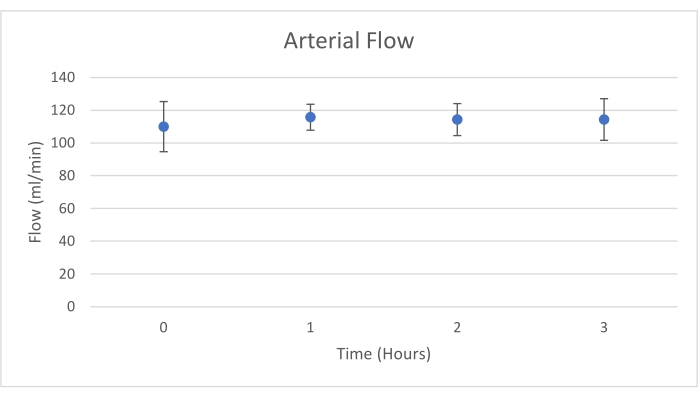
Figure 4. Mean arterial flow with standard deviation (mL/min). Please click here to view a larger version of this figure.
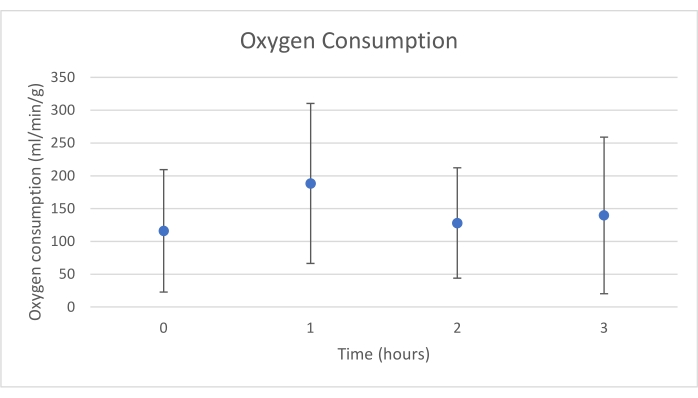
Figure 5. Mean oxygen consumption with standard deviation (mL/min/g). Please click here to view a larger version of this figure.
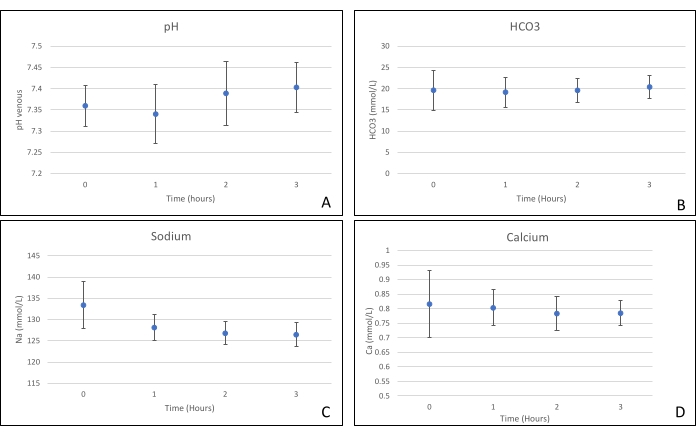
Figure 6. (A) Mean pH, (B) HCO3, (C) sodium, and (D) calcium measurements with standard deviations. Please click here to view a larger version of this figure.
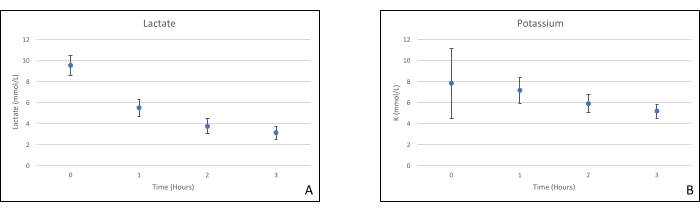
Figure 7. (A) Mean lactate and (B) potassium measurements with standard deviations. Please click here to view a larger version of this figure.
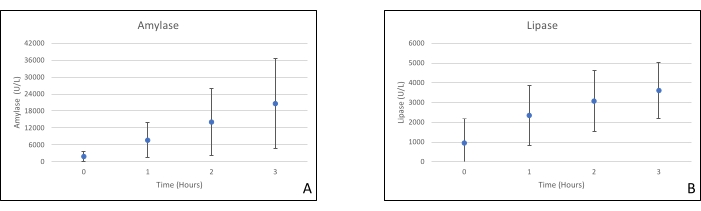
Figure 8. (A) Mean amylase and (B) lipase measurements with standard deviations. Please click here to view a larger version of this figure.
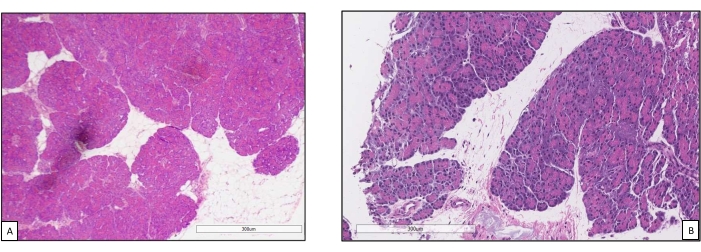
Figure 9. Core biopsies before and after perfusion. (A) Normal pancreatic parenchyma before machine perfusion18. (B) Post perfusion biopsy with good preservation of pancreatic acini and islet cells. Please click here to view a larger version of this figure.
| Ingredient | Amount |
| Ringer’s lactate | 260 mL |
| Steen Solution | 195 mL |
| Washed erythrocytes | 162.5 mL |
| Double Reverse Osmosis Water (DRO) | 35 mL |
| Heparin (10000 IU / 10 mL) | 1.3 mL |
| Sodium bicarbonate (8.4%) | 10.4 mL |
| Calcium gluconate (10%) | 1.3 mL |
| Metylprednisolone (Solu-Medrol) | 325 mg |
| Aprotinin | 15 mg |
Table 1. Perfusate composition.
| Weight before | Weight after | Gain | % difference | |
| Case 1 | 244 g | 240 g | -4 g | -1.63 |
| Case 2 | 154 g | 164 g | 10 g | 6.49 |
| Case 3 | 184 g | 245 g | 61 g | 33.15 |
| Case 4 | 190 g | 226 g | 36 g | 18.94 |
| Case 5 | 198 g | 307 g | 109 g | 55.05 |
| Case 6 | 205 g | 315 g | 107 g | 51.44 |
| Case 7 | 193 g | 256 g | 63 g | 32.64 |
Table 2. Weight before and after perfusion.
Supplemental File: Custom made pancreas chamber for perfusion. Designed in collaboration with the Machine Shop of the Medical Physics - Radiation Medicine Program at Princess Margaret Cancer Centre. Please click here to download this File.
Discussion
This study demonstrates that stable NEVPP can be achieved for pancreas allografts with minimal histological damage after 3 h of perfusion with the setup previously presented. Perfusion parameters like arterial flow, pressure, pH, HCO3, and Na remain stable during perfusion, and we observed a decrease and stabilization of K and lactate.
It is of critical importance to manipulate the graft as little as possible during procurement, back table preparation, and perfusion. It is also very important to keep tight control of the arterial pressure. Since the pancreas is a low-pressure organ, an increase in the pressure may cause irreversible damage to the organ.
Back-table preparation for this study is different from human grafts preparation (Figure 2A). Since the pancreas was the only organ procured from the pigs, we were able to take the portion of the aorta that includes the celiac trunk and superior mesenteric artery. As for the portal vein, an extension using iliac vein was performed. In case of human grafts, back-table preparation will have to be done in the same manner as it is done for transplantation, using iliac grafts for arterial reconstruction and portal lengthening19.
This method might be limited by the complexity of the setup. We decided to add a dialysis cassette after noticing severe edema of the graft when done without it. A custom-made organ chamber was also constructed for these experiments which contained an external heating source that proved to be instrumental for the optimal perfusion of the grafts.
There are few studies describing normothermic ex vivo pancreas perfusion. In most of these studies, edema appears to be the major limiting factor. To our knowledge, this method is the only report of using a dialysis cassette to control edema.
Normothermic ex vivo perfusion for the pancreas is still in its infancy compared to other organs. Current protocols are focusing on extended criteria donors (DCD), perfusate improvement, longer perfusion times, and biomarkers to assess graft damage during perfusion. Amylase and lipase levels don't seem to be reliable markers, since we are using a closed system, and don't seem to correlate with the histopathology20. So far, our group has also managed to transplant pancreas allografts after perfusion with good results18.
With continued improvements in this technology, we hope this technology will be applicable to clinical transplantation and allow for assessment and repair of pancreas allografts. This will hopefully ultimately result in more graft utilization, decreased waiting time for patients, and better patient outcomes
Disclosures
The authors do not have anything to disclose.
Acknowledgements
None.
Materials
| Name | Company | Catalog Number | Comments |
| Alburex 5 | CSL Behring AG | 187337 | 25 g of Albumin (human) in 500 mL of buffered diluent |
| Aprotinin from bovine lung | Sigma-Aldrich | A1153 | |
| Belzer UW Cold storage solution | Bridge to life Ltd | 4055 | |
| Calcium gluconate (10%) | Fresenius Kabi Canada Ltd (Toronto, ON) | C360019 | |
| Composelect (blood collection bags) | Fresenius Kabi Canada Ltd | PQ31555 | |
| Epoprostenol | GlaxoSmithKline Inc. | 218761 | |
| Heart lung machine, Stöckert S3 | Sorin Group Canada Inc. | Custom made | Centrifugal pump, roller pump, control panel (sensors for pressure, flow, temperature, bubbles, and level), oxygen blender, heater unit |
| Hemoflow, Fresenius Polysulfone | Fresenius Medical Care North America | 0520165A | |
| Heparin (10000 IU/10 mL) | Fresenius Kabi Canada Ltd | C504710 | |
| Lactated Ringer's solution | Baxter | JB2324 | |
| Neonatal cardiopulmonary bypass techonolgy | Sorin Group Canada Inc | Custom made | Dideco perfusion tubing systems, centrifugal blood pump (Revolution), arterial blood filter, microporous hollow fibre memebrane oxygenator), cannulas |
| Pancreas chamber | Custom made | With external heater | |
| Percutaneous Sheath Introducer Set with Integral Hemostasis Valve/side Port for use with 7-7.5 Fr Catheters | Arrow International LLC | SI-09880 | |
| Sodium bicarbonate (8.4%) | Fresenius Kabi Canada Ltd | C908950 | |
| Solu-Medrol | Pfizer Canada Inc. | 52246-14-2 | |
| Steen | XVIVO | 19004 | |
| Urethral catheter | Bard Inc | 86020 | 20 Fr, malecot model drain |
| Verapamil | Sandoz Canada Inc. | 8960 |
References
- . National Diabetes Statistics Report | Diabetes | CDC Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html (2022)
- Shyr, Y. M., Wang, S. E., Chen, S. C., Shyr, B. U. Reappraisal of pancreas transplantation. Journal of the Chinese Medical Association JCMA. 82 (7), 531-534 (2019).
- Dholakia, S., et al. Pancreas transplantation: past, present, future. American Journal of Medicine. 129 (7), 667-673 (2016).
- Johnson, P., Sharples, E., Sinha, S., Friend, P. J. Pancreas and islet transplantation: pancreas and islet transplantation in diabetes mellitus. Transplantation Surgery. , 205-217 (2021).
- Kopp, W. H., et al. Pancreas transplantation with grafts from donors deceased after circulatory death: 5 years single-center experience. Transplantation. 102 (2), 333-339 (2018).
- Cypel, M., et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. The New England Journal of Medicine. 364 (15), 1431-1440 (2011).
- Nasralla, D., et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 557 (7703), 50-56 (2018).
- Selzner, M., et al. Normothermic ex vivo liver perfusion using steen solution as perfusate for human liver transplantation: First North American results. Liver Transplantation: Official Publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 22 (11), 1501-1508 (2016).
- Hosgood, S. A., Thompson, E., Moore, T., Wilson, C. H., Nicholson, M. L. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. The British Journal of Surgery. 105 (4), 388-394 (2018).
- Urbanellis, P., et al. Normothermic ex vivo kidney perfusion improves early dcd graft function compared with hypothermic machine perfusion and static cold storage. Transplantation. 104 (5), 947-955 (2020).
- Barlow, A. D., et al. Use of ex vivo normothermic perfusion for quality assessment of discarded human donor pancreases. American Journal of Transplantation: Official Journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 15 (9), 2475-2482 (2015).
- Kumar, R., et al. Ex vivo normothermic porcine pancreas: A physiological model for preservation and transplant study. International journal of surgery. 54, 206-215 (2018).
- Hamaoui, K., et al. Development of pancreatic machine perfusion: translational steps from porcine to human models. The Journal of Surgical Research. 223, 263-274 (2018).
- Prudhomme, T., et al. Successful pancreas allotransplantations after hypothermic machine perfusion in a novel diabetic porcine model: a controlled study. Transplant International: Official Journal of the European Society for Organ Transplantation. 34 (2), 353-364 (2021).
- Kaths, J. M., et al. Normothermic ex vivo kidney perfusion for the preservation of kidney grafts prior to transplantation. Journal of Visualized Experiments: JoVE. (101), e353 (2015).
- Graham, A. S., Ozment, C., Tegtmeyer, K., Lai, S., Braner, D. A. V. Central venous catheterization. The New England Journal of Medicine. 356 (21), 21 (2009).
- Swindle, M. M. . Swine in the Laboratory Surgery, Anesthesia, Imaging, and Experimental Techniques. , (2015).
- Mazilescu, L. I., et al. Normothermic ex situ pancreas perfusion for the preservation of porcine pancreas grafts. American Journal of Transplantation. , (2022).
- Fridell, J. A., et al. Preparation of the pancreas allograft for transplantation. Clinical transplantation. 25 (2), (2011).
- Nassar, A., Liu, Q., Walsh, M., Quintini, C. Normothermic ex vivo perfusion of discarded human pancreas. Artificial Organs. 42 (3), 334-335 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved