A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Ceramic Omnidirectional Bioprinting in Cell-Laden Suspensions for the Generation of Bone Analogs
In This Article
Summary
This protocol describes a 3D printing technique to fabricate bone-like structures by depositing a calcium phosphate ink in a gelatin-based granular support. Printed bone analogs are deposited in freeform, with flexibility for direct harvesting of the print or crosslinking within a living cell matrix for multiphasic constructs.
Abstract
Structurally, bone tissue is an inorganic-organic composite containing metabolically active cells embedded within a hierarchical, highly mineralized matrix. This organization is challenging to replicate due to the heterogeneous environment of bone. Ceramic omnidirectional bioprinting in cell-suspensions (COBICS) is a microgel-based bioprinting technique that uniquely replicates the mineral and cellular structure of bone. COBICS prints complex, biologically relevant constructs without the need for sacrificial support materials or harsh postprocessing steps (e.g., radiation and high-temperature sintering), which are two of the biggest challenges in the additive manufacturing of bone mimetic constructs. This technique is enabled via the freeform extrusion of a novel calcium phosphate-based ink within a gelatin-based microgel suspension. The yield-stress properties of the suspension allow deposition and support the printed bone structure. UV crosslinking and nanoprecipitation then "lock" it in place. The ability to print nanostructured bone-mimetic ceramics within cell-laden biomaterials provides spatiotemporal control over macro- and micro-architecture and facilitates the real-time fabrication of complex bone constructs in clinical settings.
Introduction
Bone has remarkable regeneration abilities as one of the few structures in the body that can heal by recreating its normal cellular composition, orientation, and mechanical strength up until a critical defect size, when endogenous healing capacity is compromised1. Bone, together with cartilage and ligament, supports and facilitates body movement, while also storing minerals and fats and producing blood cells. As a hard, dense connective tissue, bone is mainly composed of an inorganic phase, water, and organic material composed primarily of collagen fibers2. Cells are embedded within this highly mineralized matrix of collagen I fibers and hydroxyapatite (HA) crystals, forming a hierarchical structure3.
The complex organization of this tissue makes the fabrication of synthetic alternatives to replicate the heterogeneous bone micro- and nano-environments exceptionally challenging3. For this purpose, a variety of materials, including bioceramics, cell-laden hydrogels, and synthetic materials have been proposed as solutions to create bone matrices. Among the scaffold fabrication techniques, 3D printing-based techniques have recently emerged and received much attention from the tissue engineering community owing to their remarkable ability to allow the fabrication of highly sophisticated and precise structures with great promise of patient-specific treatment4,5,6. Hydrogels have been the most popular choice of matrix mimics and bio-inks since they can be printed together with cells and bioactive molecules, generating functional constructs6. However, hydrogels lack the functional properties of bone, such as mechanical strength and a highly calcified, inorganic phase containing metabolically active cells.
3D printed ceramic scaffolds typically require postprocessing steps, including sintering, high-temperature treatments, or using harsh chemicals that must be thoroughly washed before in vitro or in vivo applications5. To address these limitations, Lode et al.7 recently developed an α-tricalcium phosphate-based paste formed by hydroxyapatite, which can be printed and set under physiological conditions. However, this material still cannot be printed together with live cells as it requires post-treatment in a humid environment and subsequent aqueous solution immersion for a long period.
Alternatively, cell-laden hydrogels with inorganic particles incorporated have been proposed as a replacement for 3D bone matrix8,9. Despite their great ability to support cell viability, they are not able to recapitulate the densely mineralized bone tissue environment. Thrivikarman et al.10 adopted a biomimetic approach in which a supersaturated calcium and phosphate medium was used with a non-collagenous protein analog to better mimic the nanoscale apatite deposition. However, their constructs still cannot generate rigid 3D constructs with micro- and macro-scale architecture resembling bone.
The present study addresses these shortcomings through the development of a printing strategy to fabricate bone-mimicking constructs, in inorganic and organic phases, that are able to integrate both cells and growth factors11. COBICS uniquely recapitulates the mineral and cellular structure of bone using a microgel-based bioprinting technique. The protocol herein describes the process of synthesizing the ceramic bone-ink and gelatin-based microgels and then combining cells that enable COBICS. The process begins with the synthesis of the main precursor material of the bone-ink. The cross-linkable hydrogel is then synthesized and formed into microgels. Lastly, the bone-ink is deposited omnidirectionally in a support bath of the microgels laden with cells (Figure 1).
The bone-ink may be printed into any suspension of microgels that have the appropriate yield-stress characteristics, that is, the ability to fluidize at a specific shear rate and subsequently support the deposited structure. Two flexible approaches have been demonstrated: a suspension consisting of gelatin microgels and a suspension consisting of gelatin methacrylate (GelMA) microgels. The former suspension dissolves when the temperature is raised to 37 °C, the freeform reversible embedding of suspended hydrogels (FRESH) technique12, while the latter can be photocrosslinked after printing, effectively "stitching" the microgels together and locking the printed bone-ink in place. The present study focuses on using GelMA as the matrix as it provides the unique advantage of being able to support cell growth with in situ printing of complex bone mimetic structures. Ultimately, this approach enables the generation of complex tissue models with high levels of biomimicry and broad implications for disease modeling, drug discovery, and regenerative engineering.
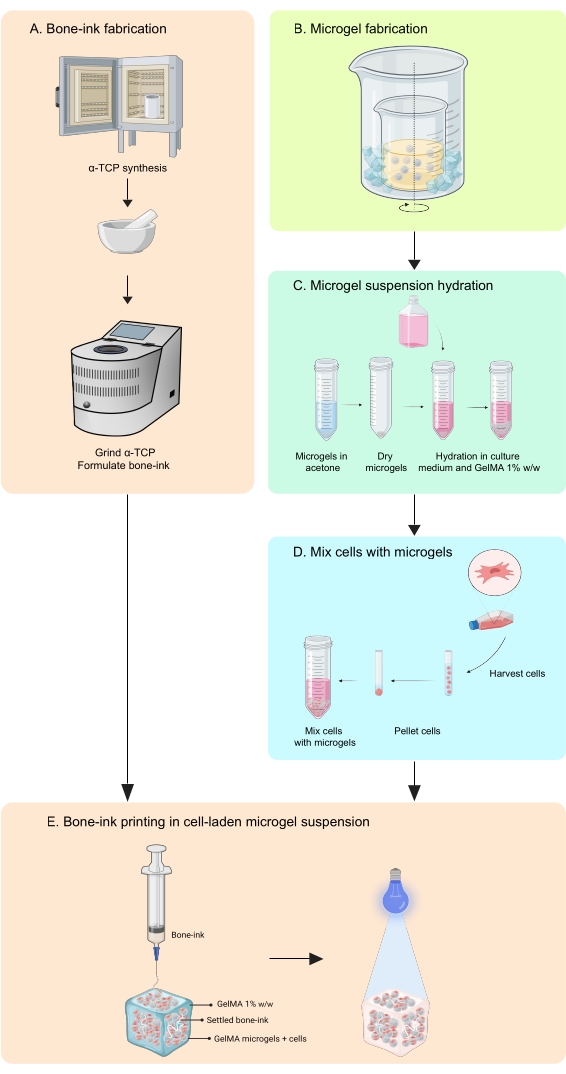
Figure 1: Schematic of the workflow. (A) The bone-ink is synthesized starting from α-tricalcium phosphate synthesis and its subsequent combination with glycerol, polysorbate 80, and ammonium phosphate dibasic. (B) GelMA microgels are fabricated by the water-in-oil emulsion method. The obtained microgels are then (C) hydrated and (D) combined with cells. Cell-microgel composites are then used as a granular bath in which the bone-ink is deposited. (E) The whole construct is then UV-crosslinked and transferred to the incubator for culture. Abbreviations: α-TCP = α-tricalcium phosphate; GelMA =gelatin methacrylate. Please click here to view a larger version of this figure.
Protocol
1. Bone-ink fabrication
- Synthesis of α-tricalcium phosphate
- Weigh out calcium hydrogen phosphate (CaHPO4) and calcium carbonate (CaCO3) powders in a 3:2 Ca:P molar ratio. Using a spatula, thoroughly homogenize the two powders.
- Add the calcium hydrogen phosphate-calcium carbonate powder mixture to a zirconia crucible such that it is no more than 75% full.
NOTE: To avoid contamination, use a new crucible or a crucible previously used to make the same material. To clean, rinse with 100% ethanol and air-dry in a fume hood until completely dry before adding the powders. - Transfer the crucible to a furnace. Heat to 1,400 °C at a rate of 5 °C/min and hold for 3 h.
- Quench the reaction by removing the crucible from the furnace and leave it atop a refractory block. Allow it to cool completely before handling.
NOTE: Use crucible tongs of an appropriate length and ensure adequate heat protection. - Use a mortar and pestle to break and grind the α-TCP cake such that the resulting granules have a maximum size of 200 µm.
NOTE: Use a standard stainless-steel sieve to ensure correct particle size. - Further grind the granules using a planetary mill in two stages. First, add 3 mm yttria-stabilized zirconia balls in a weight ratio of 8:1 balls:powder, then 100% ethanol in a weight ratio of 3:1 ethanol:powder. Secure the lid and grind for 2 h at 180 rpm.
- Collect the suspension and separate the balls, using 100% ethanol for washing.
- Dry the suspension in an oven at 120 °C for 24 h.
- Add the dried powder to milling jars with 1 mm zirconia balls and 100% ethanol in the same weight ratios as in the first stage. Grind for 2 h at 180 rpm, separate, and dry.
NOTE: The entire synthesis procedure is represented in Figure 1A.
- Formulation of bone-ink
- To make the bone-ink, add 2 g of α-TCP powder to a ball mill jar that contains 630 µL of glycerol and 130 µL of polysorbate 80 whilst continuously stirring with a spatula.
- Add 100 mg of ammonium phosphate dibasic ((NH4)2HPO4, APD) and stir to combine.
NOTE: Excessive residue of liquid phases left on the spatula will result in a ratio imbalance of the ink components and, hence, the kinetics of setting. - Add a 25 mm zirconia ball, secure the lid, and place it inside a planetary mill for 60 min at 180 rpm, pausing halfway to scrape down the sides of the jar with a spatula.
- Using a spatula, load the ink into a 1 mL syringe. Wrap adequately to avoid contact with moisture. Store at −20 °C if not used immediately.
- Bone-ink microstructure characterization
- Print the bone-ink in deionized water and allow to set for 5 min.
- Wash the sample 3x with 100% ethanol and allow it to dry completely.
- Coat with a thin layer (15 nm thickness) of gold.
- Capture micrographs using a field emission scanning electron microscope at an acceleration voltage of 5 kV.
2. Fabrication of microgel suspensions for printing
- Synthesis of GelMA
NOTE: This procedure has been tested for batch sizes consisting of 10 g and 20 g of gelatin. This method details measurements for a batch using 10 g.- Make a 10% w/w solution of gelatin type A (porcine, Bloom strength 300) in 1x phosphate-buffered saline (PBS) by weighing out 10 g of gelatin and adding it to a conical flask with 90 mL of PBS. Heat to 50 °C while stirring until the gelatin is completely dissolved.
- Add 5.796 mL of methacrylic anhydride. Place a rubber cap on the conical flask and continue stirring in the dark at 50 °C for 90 min.
CAUTION: Methacrylic anhydride is toxic if inhaled or swallowed and is a skin and eye irritant. Handle only inside a fume hood and use appropriate PPE. - Quench the reaction by diluting the conical flask's contents twofold with PBS.
- Decant into 50 mL tubes and centrifuge at 3,000 × g at room temperature for 3 min to remove unreacted methacrylic anhydride.
- Dialyze the supernatant inside 14 kDa cutoff cellulose dialysis tubes against deionized water at 40 °C for 5 days while gently stirring. Replace the deionized water every day.
- Prepare for storage by decanting into 50 mL tubes, securing the cap, and placing it in the refrigerator for 12 h. Store in a refrigerator for up to 7 days.
- Freeze using liquid nitrogen and immediately lyophilize for 5 days at −54 °C and 0.4 mbar.
NOTE: Ensure that the tubes contain no more than 40 mL of liquid when freezing. Once frozen, replace the cap with a covering that allows gas exchange such as a delicate task wipe secured with an elastic band. - Store the resulting foam in a freezer at −20 °C until required for microgel suspension synthesis.
- Synthesis of GelMA microgels
NOTE: The microgels are synthesized using a water-in-oil emulsion method13 (Figure 2). This method has been tested for GelMA solution volumes of 1-10 mL. The same protocol can be used to synthesize gelatin microgels used to print standalone bone-prints.- Make a 10% w/w GelMA solution in PBS by weighing the lyophilized GelMA, adding it to a tube with PBS, and heating in a water bath at 50 °C until fully hydrated.
- Add 37 mL of oil per 1 mL of GelMA solution to a beaker, ensuring it is no more than 65% full.
- Set up a double-beaker system on a hot plate with magnetic stirring by placing the beaker containing oil inside a larger beaker.
NOTE: The size of the two beakers should be such that ice can be easily dropped into the space between their walls. The setup is shown in Figure 2. - Heat to 40 °C while stirring.
NOTE: Ensure the vortex is not turbulent and has a depth of approximately 1/3 of the height of the oil in the beaker. - Load the GelMA solution into a syringe and add it dropwise into the stirring oil through a 0.45 µm filter. Allow the emulsion to equilibrate for 10 min.
- Reduce the temperature of the emulsion to 15 °C to thermally stabilize the spheres by adding crushed ice into the space between the two beakers.
- Add acetone to the spinning emulsion in a volume ratio of 1:11 GelMA solution to acetone.
NOTE: Add the acetone gently through a funnel to avoid disrupting the emulsion. Stir for 60 min. - Decant the contents of the beaker into 50 mL tubes, making sure to wash the walls of the beaker with acetone. Leave for 20 min to allow the dehydrated microgels to settle to the bottom.
- Discard the supernatant and wash at least 2x with acetone.
NOTE: The supernatant should be clear. - Consolidate into one tube, top up with acetone, and sonicate for 10 s. Wash 2x with acetone.
- Store in acetone at room temperature until required for printing.
- Preparing the GelMA microgel suspension for printing
- Prepare a 1% w/w solution of GelMA in Dulbecco's Modified Eagle Medium (DMEM) by weighing lyophilized GelMA into a tube, adding DMEM, and heating in a water bath at 50 °C until fully hydrated.
- Evaporate the acetone from the dehydrated microgels and weigh the resulting powder into a tube. Add acetone and transfer to a sterile environment.
- To form the microgel suspension, evaporate the acetone and add DMEM, 1% w/w GelMA solution in DMEM, and 2.5% w/w lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) initiator solution to achieve a final packing fraction of 30%. Allow to fully hydrate for at least 12 h at room temperature. Store in a refrigerator for up to 7 days. Allow to come up to room temperature before use.
NOTE: The volumes of these reagents are based on the dry weight of the microgels and can be calculated using the equations in Table 1.
| Equation | |
| x = weight of dry microgels (mg) | |
| Volume of 1% w/w GelMA in DMEM, a (µL) | a = 21.93x |
| Volume of DMEM, b (µL) | b = 8.773x |
| Volume of 2.5% w/w LAP solution, c (µL) | c = 0.6267x |
| Total volume of microgel suspension produced (µL) | a + b + c |
Table 1: Equations for calculating the volumes of reagents required to hydrate the GelMA microgel suspensions. Abbreviations: GelMA = gelatin methacrylate; LAP = lithium phenyl-2,4,6-trimethylbenzoylphosphinate.
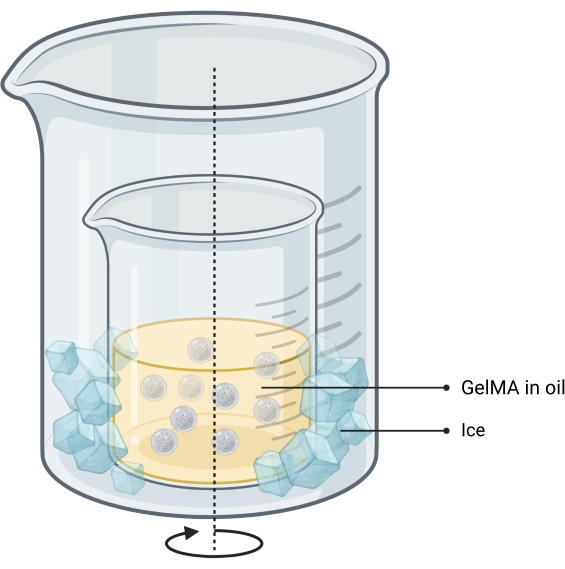
Figure 2: Schematic of the oil-emulsion method used for microgel synthesis. The double-beaker setup shows a beaker containing the stirring (indicated by arrow) emulsion placed inside a larger beaker to allow cooling. Abbreviation: GelMA = gelatin methacrylate Please click here to view a larger version of this figure.
3. Printing bone-ink into cell suspensions
NOTE: Gelatin-based microgels support the adhesion of many different cell types, which makes this approach amenable to single and multiple cells within the microgel matrix. This protocol describes the procedure for using adipose-derived mesenchymal stem cells (ADSCs), as this is a popular and robust cell type for musculoskeletal tissue engineering.
- Culture the ADSCs in low-glucose DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37 °C and 5% CO2 until confluent.
- Detach the ADSCs from the tissue culture flask by removing the medium, washing with sterile PBS, and incubating at 37 °C and 5% CO2 with 0.25% trypsin for 3 min.
- Pellet the cells by centrifuging at 150 × g at room temperature for 5 min.
- Count the cells and calculate 5 × 105 cells for each 1 mL of GelMA microgels. Allocate the required volume of cell suspension to a separate tube and pellet as above.
- Carefully remove as much supernatant as possible using a pipette, leaving only the cell pellet. Add the required volume of microgel suspension to the pellet and aspirate gently to ensure even cell distribution.
NOTE: If there are excess air bubbles in the suspension, gently centrifuge to remove and pipette up and down to redistribute the cells. - Load the cell-laden microgel suspension into a reactor using a pipette.
NOTE: In the present study, 10 mm x 10 mm x 3 mm reactors with a volume capacity of 100 µL were 3D printed. - Deposit bone-ink using a 1 mL syringe fitted with a 23 G needle.
NOTE: This can be done with a 3D printer either by retrofitting an extrusion system that allows printing directly from the 1 mL syringe or by loading the bone-ink directly into the printer's extrusion cartridge (Figure 3). - Crosslink the cell- and bone-ink-laden GelMA microgel construct with a UV-crosslinker lamp (405 nm) for 90 s. Immediately transfer to an appropriately sized well plate and cover with complete DMEM.
NOTE: The aforementioned 3D-printed reactors fit inside 24-well cell culture plates. - Incubate at 37 °C and 5% CO2. Replace the culture medium after 24 h, then every 48-72 h as required.
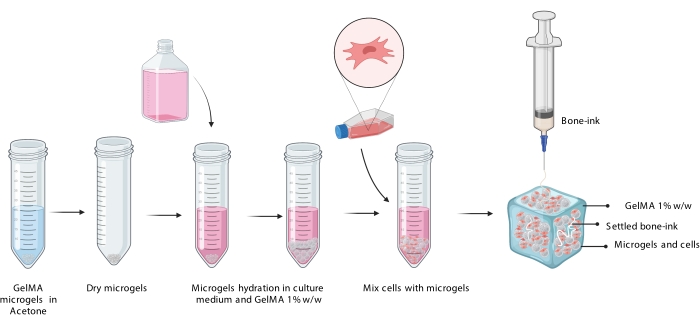
Figure 3: Schematic representation of the COBICS procedure showing the hydration of microgels, incorporation of cells, and subsequent printing of bone-ink in the cell-laden microgel suspension. Abbreviation: COBICS = ceramic omnidirectional bioprinting in cell-suspensions; GelMA = gelatin methacrylate. Please click here to view a larger version of this figure.
4. Cell viability and proliferation assessment
- To assess bone-ink cytotoxicity, keep cell-laden COBICS constructs in complete culture medium. Perform a Live Dead assay at 24 h, 72 h, and 120 h (or relevant time points).
- At each time point, wash the constructs with PBS, then add a solution of phenol-free DMEM containing 4 mM calcein and 2 mM ethidium bromide. Incubate for 1 h at 37 °C and 5% CO2.
- Wash with PBS and transfer to a glass-bottomed dish for imaging with a confocal microscope at Ex/Em spectra of 494/517 nm and 528/617 nm.
Results
COBICS prints complex, biologically relevant constructs without the need for sacrificial support materials or harsh postprocessing steps (e.g., radiation and high-temperature sintering) which are two of the biggest challenges in the additive manufacturing of bone mimetic constructs. To demonstrate COBICS formation of complex bone structures and the co-printing of cells in microgel suspensions, representative images of bone-like composites made of the bone-ink were taken, and a semi-quantitative analysis of ADSC viability...
Discussion
The 3D printing technique COBICS was developed to enable the fabrication of mineralized bone-like structures via extrusion into a crosslinkable microgel suspension containing live cells. The technique has been applied to a degradable microgel suspension, and cells show good viability, spreading, and osteogenic differentiation ability within the system11. A key determinant of the success of constructs created using this technique is the proper synthesis of the α-TCP-based bone-ink. First, the ...
Disclosures
The authors declare that they do not have any conflicts of interest to disclose.
Acknowledgements
The authors would like to acknowledge the National Health and Medical Research Council (Grant no. GNT1111694 and GNT1141602) and the Australian Research Council (Grant no. FT180100417, FL150100060, and CE14100036). The authors would like to acknowledge the Biomedical Imaging Facility at the University of New South Wales. Figures were created with Biorender.com, Adobe Photoshop, and Adobe Illustrator and have been exported under a paid subscription.
Materials
| Name | Company | Catalog Number | Comments |
| 3D Printer Extruder | Hyrel3D | EMO-25 | |
| 50 mL centrifuge tubes | Falcon | BDAA352070 | |
| Absolute Ethanol 100% Denatured | Chem-Supply | ||
| Acetone | Chem-Supply | 154871 | |
| Alumina crucible | Coors | ||
| Ammonium phosphate dibasic (NaHPO4) | Sigma | A5764 | |
| Autodesk Fusion 360 | Autodesk | ||
| Biosafety cabinet level 2 | |||
| Calcium carbonate | Sigma | 239216 | |
| Calcium hydrogen phosphate (CaHPO4) | Sigma | C7263 | |
| Cell culture flasks | Corning | various volumes used | |
| Cellulose Dialysis Tubes, 14 kDa cut-off | Sigma | D9777 | |
| Centrifuge | Eppendorf | 5430R | |
| Centrifuge | Sigma | 3-16KL | |
| Dispensing Tip, 23 G | Nordson | 7018302 | |
| DMEM, low glucose, pyruvate | Thermo FIsher | 11885084 | |
| DPBS, no calcium, no magnesium | Thermo FIsher | 14190144 | |
| Elevator furnace | Labec | ||
| Engine HR Multihead Printer | Hyrel3D | ||
| Fetal Bovine Serum | Bovogen | ||
| Gelatin type A, from porcine skin | Sigma | G2500 | |
| General Purpose Stainless Steel Tips | Nordson EF | ||
| Glycerol | Sigma | G9012 | |
| Human adipose derived stem cells | ATCC | PCS-500-011 | |
| LSM 800 Confocal Microscope | ZEISS | ||
| Lyophilizer (Alpha 1-4 LDplus) | Christ | 101541 | |
| Magnetic hot plate and stirrer | |||
| Methacrylic anhydride | Sigma | 276685 | |
| Mini 2 Desktop 3D Printer | LulzBot | ||
| Parafilm sealing film | Parafilm | PM996 | |
| Penicillin-Streptomycin | Thermo FIsher | 15140122 | |
| Planetary ball mill | |||
| Planetary ball mill jar | |||
| Polyoxyethylenesorbitan monooleate Tween-80 | Sigma | P6224 | |
| Scanning electron microscope | FEI Nova NanoSEM 450 FE-SEM | ||
| Science Kimwipes Delicate Task Wipers | Kimtech | 18813156 | |
| Stainless steel standard test sieve | |||
| Sunflower Oil | Community Co | ||
| Trypsin-EDTA 0.25% phenol red | Thermo FIsher | 25200056 | |
| ZEN Microscope Software | ZEISS | ||
| Live/Dead viability/ cytotoxicity kit for mammalian cells | Invitrogen | L3224 | |
| DMEM, low glucose, no phenol red | Thermo Fisher | 11054020 |
References
- Bates, P., Ramachandran, M. Bone injury, healing and grafting. Basic Orthopaedic Sciences. The Stanmore Guide. , 123-134 (2007).
- Lin, X., et al. The bone extracellular matrix in bone formation and regeneration. Frontiers in Pharmacology. 11, 757 (2020).
- Reznikov, N., et al. A materials science vision of extracellular matrix mineralization. Nature Reviews Materials. 1, 16041 (2016).
- Kang, H. W., et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nature Biotechnology. 34 (3), 312-319 (2016).
- Lin, K., et al. 3D printing of bioceramic scaffolds-Barriers to the clinical translation: From promise to reality, and future perspectives. Materials. 12 (17), 2660 (2019).
- Qu, M., et al. Multi-dimensional printing for bone tissue engineering. Advanced Healthcare Materials. 10 (11), 2001986 (2021).
- Lode, A., et al. Fabrication of porous scaffolds by three-dimensional plotting of a pasty calcium phosphate bone cement under mild conditions. Journal of Tissue Engineering and Regenerative Medicine. 8 (9), 682-693 (2014).
- Bernal, P. N., et al. Volumetric bioprinting of complex living-tissue constructs within seconds. Advanced Materials. 31 (42), 1904209 (2019).
- Diloksumpan, P., et al. Combining multi-scale 3D printing technologies to engineer reinforced hydrogel-ceramic interfaces. Biofabrication. 12 (2), 025014 (2020).
- Thrivikraman, G., et al. Rapid fabrication of vascularized and innervated cell-laden bone models with biomimetic intrafibrillar collagen mineralization. Nature Communications. 10 (1), 3520 (2019).
- Romanazzo, S., et al. Synthetic bone-like structures through omnidirectional ceramic bioprinting in cell suspensions. Advanced Functional Materials. 31 (13), 2008216 (2021).
- Hinton, T. J., et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Science Advances. 1 (9), 1500758 (2015).
- Phromsopha, T., Baimark, Y. Preparation of starch/gelatin blend microparticles by a water-in-oil emulsion method for controlled release drug delivery. International Journal of Biomaterials. 2014, 829490 (2014).
- Moreno, D., et al. Solid-state synthesis of alpha tricalcium phosphate for cements used in biomedical applications. Boletín de la Sociedad Española de Cerámica y Vidrio. 59 (5), 193-200 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved