A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Pipeline to Investigate the Structures and Signaling Pathways of Sphingosine 1-Phosphate Receptors
In This Article
Summary
S1P exerts its diverse physiological effects through the S1P receptors (S1PRs) subfamily. Here, a pipeline is described to expound on the structures and function of S1PRs.
Abstract
Lysophospholipids (LPLs) are bioactive lipids that include sphingosine 1-phosphate (S1P), lysophosphatidic acid, etc. S1P, a metabolic product of sphingolipids in the cell membrane, is one of the best-characterized LPLs that regulates a variety of cellular physiological responses via signaling pathways mediated by sphingosine 1-phosphate receptors (S1PRs). This implicated that the S1P-S1PRs signaling system is a remarkable potential therapeutic target for disorders, including multiple sclerosis (MS), autoimmune disorders, cancer, inflammation, and even COVID-19. S1PRs, a small subset of the class A G-protein coupled receptor (GPCR) family, are composed of five subtypes: S1PR1, S1PR2, S1PR3, S1PR4, and S1PR5. The lack of detailed structural information, however, impedes the drug discovery targeting S1PRs. Here, we applied the cryo-electron microscopy method to solve the structure of the S1P-S1PRs complex, and elucidated the mechanism of activation, selective drug recognition, and G-protein coupling by using cell-based functional assays. Other lysophospholipid receptors (LPLRs) and GPCRs can also be studied using this strategy.
Introduction
Sphingosine-1-phosphate (S1P), a metabolic product of sphingolipids in the cell membrane, is a ubiquitous lysophosphatidic signaling molecule that involves various biological activities, including lymphocyte trafficking, vascular development, endothelial integrity, and heart rate1,2,3. S1P exerts its diverse physiological effects through five S1P receptor subtypes (S1PRs 1-5); S1PRs are found in a variety of tissues and exhibit unique preferences for downstream G proteins4,5. S1PR1 is primarily coupled with the Gi protein, which subsequently inhibits cAMP production; S1PR2 and S1PR3 are coupled with Gi, Gq, and G12/13, and S1PR4 and S1PR5 transduce signal through Gi and G12/136.
S1P-S1PR signaling is a critical therapeutic target for multiple diseases, including autoimmune disorders7, inflammation8, cancer9, and even COVID-1910. In 2010, fingolimod (FTY720) was licensed as a first-in-class drug targeting S1PRs to treat relapse multiple sclerosis (MS)11. However, it is capable of binding to all S1PRs except S1PR2, while nonspecific binding to S1PR3 results in edema of the cerebral cortex, vascular and bronchial constriction, and lung epithelial leakage12. As an alternate strategy for increasing therapeutic selectivity, subtype-specific ligands for the receptor have been produced. Siponimod (BAF312) was approved in 2019 for the relapse MS treatment13; it effectively targets S1PR1 and S1PR5, whereas it has no affinity for S1PR3, exhibiting fewer side effects in clinical practice14. In 2020, the US Food and Drug Administration authorized ozanimod for MS therapy15. It has been reported that ozanimod holds a 25-fold greater selectivity for S1PR1 than for S1PR516. Notably, in the context of the current COVID-19 pandemic, it has been discovered that agonist drugs targeting S1PRs may be utilized to treat COVID-19 by using immunomodulatory therapy techniques17. In comparison with fingolimod, the ozanimod has shown superiority in lowering symptoms in COVID-19 patients and is now undergoing clinical trials10. Understanding the structural basis and function of S1PRs lays a significant foundation for developing a drug that selectively targets S1PRs18.
Many techniques are used to investigate the structural information of biomacromolecules, including X-ray crystallography, nuclear magnetic resonance (NMR), and electron microscopy (EM). As of March 2022, there are more than 180,000 structures deposited on the Protein Databank (PDB), and most of them have been resolved by X-ray crystallography. However, with the first near-atomic resolution structure of TPRV1 (3.4 Å resolution) reported by Yifan Cheng and David Julius in 201319, cryo-electron microscopy (cryo-EM) has become a mainstream technique for protein structures, and the total number of EM PDB structures was more than 10,000. The critical breakthrough areas are the development of new cameras for imaging known as direct electron detection cameras and new image processing algorithms. Cryo-EM has revolutionized structure biology and structure-based drug discovery in the past decade20. As understanding how macromolecular complexes fulfill their complicated roles in the living cell is a central theme in biological sciences, cryo-EM has the potential to reveal conformations of dynamic molecular complexes, particularly for transmembrane proteins21. G-protein coupled receptors (GPCRs) are the largest superfamily of transmembrane proteins and the targets of more than 30% of currently marketed pharmaceuticals22. The development of cryo-EM has contributed to a burst of high-resolution structures of GPCR-G protein complexes, enabling structural determination for 'intractable' targets that are still not accessible to X-ray crystallographic analysis in drug design23. Hence, the cryo-EM application provides a chance to determine the three-dimensional structure of GPCRs in near-native conditions at close to atomic resolution24. Advancements in cryo-EM make it possible to visualize mechanistic underpinnings of GPCR stimulation or inhibition, and further benefit in uncovering the novel binding sites for GPCR-targeted drug creation25.
Relying on the tremendous strides of cryo-EM technology, we have identified structures of agonized S1PR1-, S1PR3-, and S1PR5-Gi signaling complexes recently26,27. In humans, S1PRs are found in various tissues and exhibit unique preferences for downstream G proteins4,5. S1PR1 is primarily coupled with the Gi protein, which subsequently inhibits 3′,5′-cyclic adenosine monophosphate (cAMP) production. S1PR3 and S1PR5 are also capable of coupling with Gi6,28. Since Gi-coupled receptor activation decreases the production of cAMP29, a Gi-inhibition cAMP assay was introduced to measure cAMP inhibition effects for capturing functional alterations26,27. Using a mutant version of Photinus pyralis luciferase wherein a cAMP-binding protein moiety has been inserted, this cAMP assay offers a simple and reliable method for monitoring GPCR activity through changes in intracellular cAMP concentration30. It is a sensitive and non-radioactive functional assay and can be applied to monitor the real-time downstream signaling of a wide range of GPCRs for drug discovery purposes31.
Here, a summary is provided of the critical methods in resolving the activation and drug recognition modes of S1PRs, primarily including cryo-EM manipulations and a Gi-inhibition cAMP assay. This article aims to provide comprehensive experimental guidance for further explorations into the structures and functions of GPCRs.
Protocol
1. Purification of S1PRs-G protein complex
- To purify the human S1PRs-G protein complex, clone the cDNAs of S1PR1 lacking C-terminal residues 338-382, the wild-type S1PR3, S1PR5 truncated with 345-398 at C-terminus, and the wild-type Gi1 into the pFastBac1 vector and the cDNAs of the wild-type Gβ1 and Gγ2 into the pFastBacdual vector (Table of Materials).
NOTE: All constructs for S1PRs also contain the haemagglutinin (HA) signal sequence followed by a Flag epitope tag at the N-terminus and a 10x his tag at C-terminus. Also, a synthetic DNA sequence (Table of Materials) for translating T4 lysozyme (T4L) was inserted into the N-terminus of S1PRs in order to facilitate receptor expression and purification. - Preparation of the baculovirus encoding S1PRs, Gi1, and Gβ1γ2
- Add the recombinant vectors to 50 µL of DH5α competent Escherichia coli (E. coli) stored in a 1.5 mL tube at -80 °C, and incubate on ice for 30 min.
- Heat-shock the cells at 42 °C for 90 s, transfer them to the ice immediately, and chill them for 2 min.
- Shake the tube at 37 °C for 3-5 h after supplementing with 300 µL of lysogeny broth (LB) medium. Plate 100 µL of cells to the LB agar plate and incubate at 37 °C by keeping in dark for 48 h.
- Inoculate the white colony into 5 mL of LB medium containing 50 µg/mL kanamycin, 10 µg/mL tetracycline, and 7 µg/mL gentamicin, and culture at 37 °C for 16 h.
- Isolate the recombinant bacmid with the plasmid miniprep kit (Table of Materials) following the manufacturer's instructions, for producing the P0 baculovirus.
NOTE: Before use, the purified bacmid was analyzed by PCR with pUC/M13 forward and reverse primers. For the PCR, number of cycles = 30 cycles, melting temperature = 58 °C, and extension time = 1 min per 1 Kb. - Prepare P0 baculovirus as described in an earlier protocol32.
- Culture the sf9 cells (ESF921 medium) in six-well plates and verify that the cells are in the log phase (1.0-1.5 x 106 cells/mL).
- Dilute 8 µL of baculovirus transfection reagent in 100 µL of Grace's medium, and incubate for 30 min at room temperature. Dilute 10 µg of the recombinant bacmid in 100 µL of Grace's medium, and mix gently. Combine the diluted bacmid with diluted baculovirus transfection reagent, mix gently, and incubate for 30 min at room temperature.
- Add the mixture (bacmid and reagent) onto the cells (Step 1.2.6.1), and plate them at 27 °C for 3 h.
- Remove the Grace's medium and replace it with 2 mL of ESF921 cell culture medium. Plate the six-well plates at 27 °C and collect ESF921 cell culture medium after 5 days post-transfection.
- Centrifuge at 500 x g at 4 °C for 10 min to remove the cells and debris. Transfer the supernatant to 2 mL tubes. This is the P0 baculovirus stock.
- Isolate P1 virus stock
- Transfer 30 mL of sf9 cells into a conical bottle, culture at 27 °C with shaking at 270 rpm, and verify that the cells reach a log phase (1.0-1.5 x 106 cells/mL).
- Add 2 mL of P0 virus stock to the bottle and shake at 270 rpm for 4 days at 27 °C.
- Transfer the cells to a 50 mL tube, centrifuge at 1,800 x g for 10 min at 4 °C to remove the cells and debris, and transfer the supernatant to 50 mL tubes. This is the P1 baculovirus stock.
- Amplify baculoviral stock
- Repeat step 1.2.7 using 50 mL of sf9 cells in the log phase (1.0-1.5 x 106 cells/mL) and 1 mL of P1 stock.
- Store the resulting P2 baculovirus stock at 4 °C, protecting it from light.
NOTE: Do not amplify the baculovirus indefinitely, as the deleterious mutants were produced with each passage.
- Expression of S1PRs-G protein complex
- Culture sf9 insect cells to reach 2.5 x 106 cells/mL density, co-infect with the P2 baculovirus encoding S1PRs, Gi1, and Gβ1γ2 at a volume ratio of 1:2:1, and culture again at 27 °C for 48 h.
- Collect the cells by centrifuging at 700 x g at 4 °C for 15 min, freeze them in liquid nitrogen, and store them at -80 °C for use.
- Protein purification
- Thaw the cell pellet obtained in step 1.3 at room temperature, and then resuspend it in lysis buffer (20 mM HEPES pH 7.5, 50 mM NaCl, 5 mM MgCl2, 5 mM CaCl2) supplemented with 100 µg/mL benzamidine, 100 µg/mL leupeptin, 100 µg/mL aprotinin, 25 mU/mL apyrase, and 10 µM agonist. Stir the cell suspension at room temperature for 2 h to induce the formation of the S1PRs-G protein complex.
NOTE: Apyrase is an ATP diphosphohydrolase. It catalyzes the removal of the gamma phosphate from ATP and the beta phosphate from ADP. - Transfer the solution to the tubes, centrifuge at 70,000 x g for 10 min, and remove the supernatant carefully. Resuspend the pellet in solubilizing buffer (20 mM HEPES pH 7.5, 100 mM NaCl, 5 mM MgCl2, 5 mM CaCl2, 0.5% (w/v) LMNG, 0.1% (w/v) CHS, 1% (w/v) sodium cholate, 10% (v/v) glycerol).
- Transfer the suspension to a glass Dounce and homogenize the pellet fully. Add 10 µM agonist, 4 mg of scFv16, 100 µg/mL benzamidine, 100 µg/mL leupeptin, 100 µg/mL Aprotinin, and 25 mU/mL apyrase to the suspension, and stir at 4 °C for 2 h.
NOTE: The steps of homogenizing the pellets are crucial for the production of the GPCR-G protein complex. - Transfer the solution to the tubes, and centrifuge at 100,000 x g for 30 min at 4 °C.
- Pre-equilibrate the flag resin with the washing buffer (20 mM HEPES pH 7.5, 100 mM NaCl, 5 mM MgCl2, 5 mM CaCl2, 10 µM agonist, 0.0375% (w/v) LMNG, 0.0125% (w/v) GDN, 0.01% (w/v) CHS).
- Transfer the supernatant to the tubes, and incubate with the flag-resin at 4 °C for 2 h.
- Load the above mixture onto the glass column and wash the column with 50 mL of washing buffer supplemented with 100 µg/mL benzamidine, 100 µg/mL leupeptin, and 100 µg/mL aprotinin.
- Elute the column with 10 mL of the elution buffer containing 20 mM HEPES pH 7.5, 100 mM NaCl, 5 mM EDTA, 200 µg/mL Flag peptide, 10 µM agonist, 0.0375% (w/v) LMNG, 0.0125% (w/v) GDN, 0.01% (w/v) CHS, 100 µg/mL benzamidine, 100 µg/mL leupeptin, and 100 µg/mL aprotinin.
- Collect the S1PRs-G protein complex and concentrate to 1 mL using a 100 kDa cut-off concentrator at 1,300 x g at 4 °C. Filter through a 0.22 µM filter, and centrifuge at 13,000 x g for 10 min at 4 °C to remove the aggregates.
- Load the S1PRs-G protein complex onto a size-exclusion chromatography (SEC) gel filtration column pre-equilibrated with the SEC buffer containing 20 mM HEPES pH 7.5, 100 mM NaCl, 10 µM agonist, 100 Μm TCEP, 0.00375% (w/v) LMNG, 0.00125% (w/v) GDN, and 0.001% (w/v) CHS at a flow rate of 0.5 mL/min at 4 °C.
- Collect the peak fractions and concentrate using a 100 kDa cut-off concentrator at 1,300 x g at 4 °C for cryo-EM.
- Thaw the cell pellet obtained in step 1.3 at room temperature, and then resuspend it in lysis buffer (20 mM HEPES pH 7.5, 50 mM NaCl, 5 mM MgCl2, 5 mM CaCl2) supplemented with 100 µg/mL benzamidine, 100 µg/mL leupeptin, 100 µg/mL aprotinin, 25 mU/mL apyrase, and 10 µM agonist. Stir the cell suspension at room temperature for 2 h to induce the formation of the S1PRs-G protein complex.
2. Electron microscopy to resolve the S1PRs structure
- Data collection
- To prepare the cryo-EM grids, hold 300-mesh Au R1.2/1.3 grids for 10 s and glow discharge for 60 s at 15 mA using a glow discharge cleaning system.
- Perform sample vitrification as described previously33,34. In the plunge-freezing Console, set the temperature to 4 °C and relative humidity to 100% for the chamber working environment. Use blot force for 0, wait time of 0 s, blot time of 2 or 3 s, and drain time of 0 s. It usually only requires 3 µL of the sample at concentrations of 5-10 mg/mL for single vitrification.
- Clip and load grids into auto grid assembly, load auto grid assembly into Nanocab, and load Nanocab into the microscope by autoloader as described previously35. Screen sample quality with EPU2 software34. Usually, the data collected in the areas of suitable ice thickness was better.
- Collect cryo-EM data as described in detail previously35. For the S1P receptor, set the defocus offset between -1.0 µm and -1.8 µm with the exposure electron dose of 50-65 e-/Å2. For the S1PR1-Gi complexes, collect movie stack automatically using EPU2 software in counting mode with the K2 detector at a total exposure time of 2 s, a recording rate of five raw frames per second, and a total dose of 56 e-/Å2 to produce 35 frames per stack.
NOTE: Usually, more than 5,000 movies are needed to rebuild the receptor structure.
- Process the data using a combination of RELION36 and cryoSPARC37 to obtain an ideal cryo-EM density map. Use RELION-3.1_gpu_ompi4 to process the data initially which involves similar operations as described previously34.
- In the Linux system terminal, enter the parent directory of the data storage directory.
- Enter the relion command in the terminal to open the RELION graphical user interface (GUI).
NOTE: If this is the first time a RELION GUI has been opened in this directory, a prompt window will pop up; click on Yes. - Click on Import in the function bar on the right side of the RELION GUI to import the raw data into the RELION.
- In the Movies/mics option, select Yes for Import raw movies/micrographs?, enter the data path in the Raw input files field (wildcards are recommended), and select Yes for Are these multi-frame movies?. Enter the pixel size of movies in the Pixel size (Angstrom) field, the microscope operating voltage (in kV) in the Voltage (kV) field, and the spherical aberration of the microscope in the Spherical aberration (mm) field. These are the parameters that were recorded at the time of data collection.
- In the Running option, modify the Queue name according to the server where the program is running (other functions also need to modify this parameter). Leave the other parameters at the default values set by RELION. Finally, when all parameters are detected correctly, click RUN! to run the program.
- Use the Motion correction function for the alignment of all frames38.
- In the I/O option, click on Browse and choose the output of Import function named movies.star as input of Input movies STAR file. Enter dose per frame in the Dose per frame (e/A2) field which equals the total dose divided by the number of frames. Select No for Save sum of power spectra?.
- In the Motion option, enter 250 for B-factor, 5.5 for Number of patches X,Y, and 2 for Group frames (ensure the dose of group >3). If data was not gain-referenced during the collection period, a gain-reference image is needed which can be obtained by acquiring a blank grid area. Select No for Use RELION's own implementation? and input the directory containing the executable file of MOTIONCOR239 in the MOTIONCOR2 executable field.
- In the Running option, choose the appropriate MPI and thread number according to the computing power of the server; here, MPI = 8 and threads = 3 were used.
- Use CTF estimation function for modulating the cryo-EM image of vitrified specimen40. In the I/O option, click on Browse and choose the output of Motion cor named corrected micrographs.star as input of Input movies STAR file. In the Gctf option, select Yes for UseGctf instead?.
- Use Subset selection function to remove micrographs with a value of rlnCtfMaxResolutin >4.
- In the I/O option, click on Browse located on the right of OR select from micrographs.star and choose the output of CtfFind named micrographs_ctf.star as input. In the Subset option, select Yes for Select based on metadata values? and enter 4 for the Maximum metadata value to remove bad data.
- Manual picking: pick some images manually for the preliminary pick and classification.
- In the I/O option, click on Browse and choose micrographs_selected.star from the previous Select directory (Step 2.2.6) as input.
- Click on RUN! (a window pops up). Click on File in the top-left corner of the new window and click on Invert selection to cancel the selection of all images. Check the leftmost selection box in the corresponding row of each entry and click on pick to check the images and select ~500 good images. Click on File > Save selection to save the selected images and close the window.
- Auto-picking: automated particle picking software packages are useful and powerful41.
- In the I/O option, click on Browse to the right of Input micrographs for autopick and choose micrographs_selected.star from the previous ManualPick directory (Step 2.2.7) as input. The Laplacian-of-Gaussian algorithm is used in the beginning, so select Yes for OR: use Laplacian-of-Gaussian.
- In the Laplacian option, set Min.diameter for LoG filter (A) to 80 and Max.diameter for LoG filter (A) to 130. In the Autopicking option, set Minimum inter-particle distance (A) to 65, and select Yes for Use GPU acceleration if GPU can be accessed.
- Extract particles for the next steps.
- In the I/O option, click on Browse to the right of micrograph STAR file and choose the micrographs_selected.star from Step 2.2.6. Click on Browse to the right of Input coordinates and choose the cords_suffix_autopick.star from Step 2.2.8.
- In the Extract option, select Yes for Rescale particles and set the Re-scaled size (pixels) to 128 to speed-up later steps.
- 2D classification for preliminary classification of particles
- In the I/O option, click on Browse to the right of Input images STAR file and choose the particles.star from Step 2.2.9. In the Optimisation option, set Number of classes to 100 and Mask diameter (A) to 140.
- In the Compute option, set Number of pooled particles to 10, enter the directory which is on a fast local drive (e.g., an SSD drive) in the Copy particle to scratch directory field, and select Yes for Use GPU acceleration? for faster processing speed.
- Subset selection for selecting good 2D results as templates to choose particles
- In the I/O option, click on Browse to the right of Select classes from model.star and choose the output of Step 2.2.10 named run_it025_model.star as input. Click on RUN!. In the pop-up window, check Sort images on and Reverse sort? and click on Display!.
- Choose good representative 2D results as a reference for Reference of the Auto-picking function.
NOTE: The selected results are boxed in red. The good and bad 2D classification results are shown later. - Right-click and select Save selected classes.
- Use the template for the second round of auto-picking. In the I/O option, click on Browse to the right of Input micrographs for autopick and choose micrographs_selected.star from Step 2.2.6. Click on Browse to the right of 2D references, choose class_averages.star from Step 2.2.11, and select No for OR: use Laplacian-of-Gaussian.
- Perform particle extraction again using coord_suffix_autopick.star from Step 2.2.12 and micrographs_selected.star from Step 2.2.6.
- Perform 2D classification again using particles.star from Step 2.2.13.
- Perform subset selection again using run_it025_optimiser.star from Step 2.2.14.
NOTE: All the 2D images with clear contours and correct shapes need to be chosen. - Perform particle extraction as follows. In the I/O option, click on Browse to the right of micrograph STAR file, choose the micrographs_selected.star from Step 2.2.6, and select Yes for OR re-extract refined particles?. Click on Browse to the right of Refined particles STAR file and choose the particles.star from Step 2.2.15.
- 3D initial model and generating a reference map: In the I/O option, click on Browse to the right of Input images STAR file and choose the particles.star from Step 2.2.16. Set Number of classes to 1, and Mask diameter (A) to 140 in the Optimisation option.
- 3D classification and generating a preliminary 3D map: In the I/O option, click on Browse to the right of Input images STAR file and choose the particles.star from Step 2.2.16. Click on Browse to the right of Reference map and choose the initial_model.mrc from Step 2.2.17. Set Number of classes to 4-6, and Mask diameter (A) to 140 in the Optimisation option.
- Mask generation: Select good 3D map(s) from Step 2.2.17 as input in the I/O option. Set the Initial binarisation threshold to 0.05 (adjust based on the output), Extend binary map this many pixels to 3, and Add soft-edge of this many pixels to 8 in the Mask option.
- Use cryoSPARC for the next processing.
- Create a new workspace and click on Job Builder for the first job.
- To import the particle stack, enter the particle path (Step 2.2.16) in the Particle meta path field and the movie's path (Step 2.2.16) in the Particle data path field.
NOTE: The parameters Accelerating Voltage (kV), Spherical Aberration (mm), and Pixel Size (Angstrom) are the same as before. Amplitude Contrast (fraction) value is 0.1. - Import 3D volumes by entering the path of the best 3D volume in Step 2.2.18 in the Volume data path and selecting Map for Type of volume being imported.
- Import mask by entering the mask path (Step 2.2.19) in the Volume data path and selecting Mask for Type of volume being imported.
- Non-uniform Refinement: Take the outputs of steps 2.2.22, 2.2.23, and 2.2.24 as input.
NOTE: This function is very useful for membrane proteins.- Drag the output of Step 2.2.22 (imported_particles) as the input of Non-uniform Refinement's particles (particle), the output of Step 2.2.23 (imported_volume_1) as input of Non-uniform Refinement's volume (volume), and output of Step 2.2.24 (imported_mask_1) as input of Non-uniform Refinement's mask (mask).
NOTE: Sometimes better results can be achieved without mask. - Click Queue to start processing.
- Drag the output of Step 2.2.22 (imported_particles) as the input of Non-uniform Refinement's particles (particle), the output of Step 2.2.23 (imported_volume_1) as input of Non-uniform Refinement's volume (volume), and output of Step 2.2.24 (imported_mask_1) as input of Non-uniform Refinement's mask (mask).
- Run Steps 2.2.18-2.2.25 for better results. Through the above series of processing, a good resolution S1PR-Gi 3D map can be obtained.
3. S1PRs-Gi mediated cAMP inhibition assay
NOTE: The S1PRs-Gi mediated cAMP inhibition experiment was divided into several parts, and the following are detailed experimental procedures. The experimental principle and the general experimental process are shown in the form of a flow chart in Figure 1.
- Plasmids construction
- Sub-clone the cDNAs of wild-type S1PR1, S1PR3, and S1PR5 into the vector pcDNA3.1+ with a HA signal sequence followed by a Flag tag at the N-terminus (Table of Materials).
NOTE: Mutations for all the receptors were generated by using the mutagenesis kit (Table of Materials).
- Sub-clone the cDNAs of wild-type S1PR1, S1PR3, and S1PR5 into the vector pcDNA3.1+ with a HA signal sequence followed by a Flag tag at the N-terminus (Table of Materials).
- Preparation of the plasmid
- Add the recombinant pcDNA3.1+ vectors or the Sensor plasmid (Table of Materials) to 50 µL of DH5α competent Escherichia coli (E. coli) stored in a 1.5 mL tube at -80 °C separately, and incubate on ice for 30 min. Heat-shock the cells at 42 °C for 90 s, transfer them to the ice immediately, and chill them for 2 min.
NOTE: The Sensor plasmid provided by the Gi-inhibition cAMP assay kit (Table of Materials) expresses a modified luciferase gene fused with a cAMP binding domain and increases the luminescence activity when cAMP is bound. - Shake the tube at 37 °C for 1 h after supplementing with 300 µL of lysogeny broth (LB) medium. Plate 100 µL of cells to the LB agar plate and incubate at 37 °C by keeping in the dark for 48 h. Inoculate the white colony into 5 mL of LB medium containing 100 µg/mL Ampicillin, and culture at 37 °C for 16 h.
- Isolate DNA using the plasmid miniprep kit (Table of Materials) following manufacturer's instructions; the plasmid was at a concentration of over 350 ng/µL with the value of A260/A280 between 1.7 and 1.9.
- Add the recombinant pcDNA3.1+ vectors or the Sensor plasmid (Table of Materials) to 50 µL of DH5α competent Escherichia coli (E. coli) stored in a 1.5 mL tube at -80 °C separately, and incubate on ice for 30 min. Heat-shock the cells at 42 °C for 90 s, transfer them to the ice immediately, and chill them for 2 min.
- Cell culture
- Plate the CHO-K1 cells in a 10 cm Petri dish, culture them in a 37 °C incubator with 5% CO2,and harvest them when the monolayer is at 80%-90% confluence.
- Aspirate the CHO-K1 cells growth medium, add 4 mL of 0.05% trypsin-EDTA pre-warmed at 37 °C onto the Petri dish gently, and incubate for 15 s. Then, add 4 mL of growth medium consisting of F12 medium + 10% FBS.
- Dislodge the cells from the Petri dish surface by gently swaying and tapping the side of the Petri dish. Fill a conical tube with cell suspension. Stir and pipette slowly to remove cell clumps gently.
- Centrifuge cells at 250 x g for 5 min at room temperature, aspirate supernatant, and resuscitate with 3 mL of PBS. Repeat this step.
- Determine cell number with the hemacytometer, and centrifuge cells at 250 x g for 5 min at room temperature.
- Aspirate PBS buffer and resuspend CHO-K1 cells with 3 mL of growth medium consisting of F12 medium and 10% FBS.
- Add 2 mL of the growth medium consisting of F12 medium and 10% FBS into each well of the six-well plate, and seed 150 µL of cell suspension into each well to keep the cells at the density of 1.5 x 105 cells/mL. Incubate the six-well plate in a 37 °C tissue culture incubator with 5% CO2 for about 24 h.
- Transient transfection
- Dilute 2 µg of DNA (Step 3.2.3) into 200 µL of transfection reagent buffer (Table of Materials). Mix by vortexing for 10 s and briefly spinning before use.
NOTE: Here, the 2 µg of DNA contains 0.5 µg of receptor vector (S1PR1, S1PR3, or S1PR5) and 1.5 µg of the Sensor plasmid. - Add 4 µL of the transfection reagent (Table of Materials), vortex for 10 s, and spin briefly before use. Incubate for 15 min at room temperature.
- Slowly drop 200 µL of transfection mix into each well (containing CHO-K1 cells) to distribute evenly. Gently shake the six-well plate to ensure thorough mixing.
- Replace the transfection medium after 4-6 h with the cell growth medium consisting of F12 medium + 10% FBS, and return the six-well plate to the incubator.
- Harvest cells 24-48 h post-transfection.
- Digest the CHO-K1 cells on the well with 500 µL of 0.05% trypsin-EDTA (pre-heated at 37 °C) for 15 s andadd 1 mL of growth medium consisting of F12 medium + 10% FBS. Dislodge the cells from the well surface by rocking and gently tapping the side of the well.
- Transfer cell suspension to a conical tube, and centrifuge at 250 x g for 5 min at room temperature. Pour off the supernatant and harvest the transfected cells.
NOTE: Before the fluorescence signal determination, check the cell surface expression levels of receptors by ELISA as described previously26.
- Dilute 2 µg of DNA (Step 3.2.3) into 200 µL of transfection reagent buffer (Table of Materials). Mix by vortexing for 10 s and briefly spinning before use.
- Equilibration with D-Luciferin-potassium salt (Table of Materials)
- Suspend the harvested cells (24-48 h post-transfection) with 3 mL of assay buffer immediately (i.e., Hank's Balanced Salt Solution (HBSS) containing 10 mM HEPES pH 7.4), with an additional 3% v/v dilution of the D-Luciferin-potassium salt.
- Add 90 µL of cell suspension per well of a 96-well plate using a multichannel pipette and mix gently.
- Incubate for 40 min at room temperature.
- Fluorescence signal determination
- Prepare in advance 10 mM stock solutions of Siponimod dissolved in DMSO and make a serial dilution using HBSS buffer containing 25 µM forskolin before ligand stimulation.
NOTE: Except for the control group without ligand, the remaining have a concentration gradient range of 10-11-10-5 mol/L. - Stimulate with 10 µL (per well) of agonist solution at different concentrations for 30 min.
- Count the luminescence signals on a microplate reader using the associated software (Table of Materials) parameters as follows. Select Luminescence for Detection Method, Endpoint for Read Type, and Luminescence Fiber for Optics Type. Set the Optics Gain to 255.
NOTE: Each measurement was repeated in at least three independent experiments, each in triplicate. - Obtain the values of the fluorescence signal, import the data into a spreadsheet program, and process the data using the nonlinear regression (curve fit) dose-response function.
- Prepare in advance 10 mM stock solutions of Siponimod dissolved in DMSO and make a serial dilution using HBSS buffer containing 25 µM forskolin before ligand stimulation.
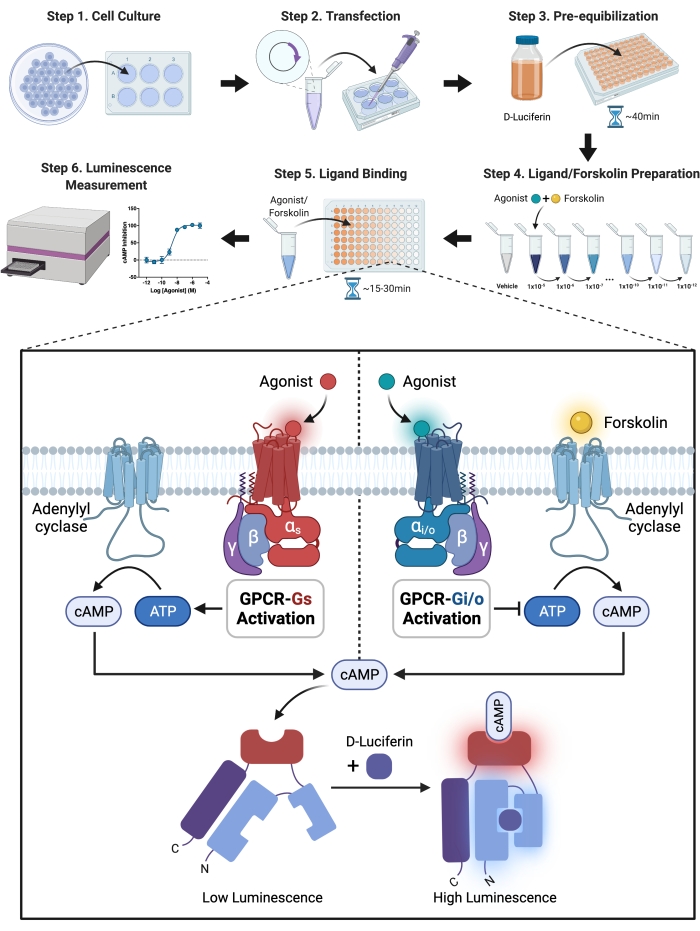
Figure 1: Schematic illustration of the experiment. A detailed step-wise guide for experimental setup and execution. In brief, the receptor and modified luciferase were transiently co-expressed in CHO-K1 cells by transfecting the receptor and Sensor plasmid into the cells with transfection reagent. The cells were suspended in HBSS solution with D-Luciferin-potassium salt, the luciferase substrate, and seeded into a 96-well plate after 24 h. To allow permeation into the cells, D-luciferin must be pre-equilibrated with the cells. The oxidative enzyme luciferase transforms luciferin to oxyluciferin and emits light. The modified luciferase, on the other hand, generates light via a chemical reaction only when bound to cAMP, and the intensity of light has a positive association with cAMP levels in cells. The levels of cAMP were regulated with GPCR activated by agonist. Gi-coupled receptors reduced the levels of cAMP, necessitating the addition of forskolin to activate the adenylyl cyclase in the Gi-inhibition cAMP experiment. Please click here to view a larger version of this figure.
Results
Before freezing the sample of S1PRs-Gi complex, the purified sample needs to be separated by size-exclusion chromatography (SEC) and analyzed with gel filtration chromatography. Figure 2 shows the S1PR3-Gi complex as an example. The peak fraction of the homogeneous GPCR-G protein complex was usually located at ~10.5 mL of the size-exclusion chromatography (Figure 2A). SDS-page analysis of the S1PR3-Gi complex (Figure 2B) reveals fou...
Discussion
This protocol describes a primary pipeline for determining the structures of S1PRs by cryo-EM and measuring the activation potency of S1PRs by Gi-mediated cAMP inhibition assay. Some steps are crucial to the experiment's success.
To purify the S1PRs-Gi complex, the quality of the virus and the health of sf9 cells should be paid more attention to. The expression of the receptor is dramatically reduced in poor sf9 cells. The health of sf9 cells was assessed by measurin...
Disclosures
Authors have no conflicts of interest.
Acknowledgements
The data of the S1PRs-Gi complex were harvested at the West China Cryo-EM Center in Sichuan University and Cryo-EM Center at the Southern University of Science and Technology (SUSTech) and processed at Duyu High-Performance Computing Center in Sichuan University. This work was supported by the Natural Science Foundation of China (32100965 to L.C., 32100988 to W.Y., 31972916 to Z.S.) and the full-time Postdoctoral Research Fund of Sichuan University (2021SCU12003 to L.C.)
Materials
| Name | Company | Catalog Number | Comments |
| 0.05% trypsin-EDTA | GIBCO | Cat# 25300054 | |
| 0.22 µM filter | Thermo Fisher Scientific | Cat# 42213-PS | |
| 100 kDa cut-off concentrator | Thermo Fisher Scientific | Cat# 88533 | |
| 6-well plate | Corning | Cat# 43016 | |
| 96-well plate | Corning | Cat# 3917 | |
| Aprotinin | Sigma-Aldrich | Cat# 9087-70-1 | |
| Apyrase | NEB | Cat# M0398S | |
| Baculovirus transfection reagent | Thermo Fisher Scientific | Cat# 10362100 | For the preparation of P0 baculovirus |
| Benzamidine | Sigma-Aldrich | Cat# B6506 | |
| CHO-K1 | ATCC | N/A | |
| CHS | Sigma-Aldrich | Cat# C6512 | |
| CryoSPARC | Punjani, A., et al.,2017 | https://cryosparc.com/ | |
| DH5α competent E.coli | Thermo Fisher Scientific | Cat# EC0112 | |
| D-Luciferin-Potassium Salt | Sigma- Aldrich | Cat# 50227 | |
| DMSO | Sigma- Aldrich | Cat# D2438 | |
| EDTA | Thermo Fisher Scientific | Cat# S311-500 | |
| ESF921 cell culture medium | Expression Systems | Cat# 96-001 | |
| Excel | microsoft | N/A | |
| F12 medium | Invitrogen | Cat# 11765 | |
| FBS | Cell Box | Cat# SAG-01U-02 | |
| Flag resin | Sigma- Aldrich | Cat# A4596 | |
| Forskolin | APExBIO | Cat# B1421 | |
| Gctf | Zhang, 2016 | https://www.mrc-lmb.cam.ac.uk/kzhang/Gctf/ | |
| GDN | Anatrace | Cat# GDN101 | |
| Gel filtration column | GE healthcare | Cat# 28990944 | |
| Gen5 3.11 | BIO-TEK | N/A | |
| Gentamicin | Solarbio | Cat# L1312 | |
| GloSensor cAMP assay kit | Promega | Cat# E1291 | Gi-inhibition cAMP assay kit |
| GloSensor plasmid | Promega | Cat# E2301 | Sensor plasmid |
| Grace’s medium | GIBCO | Cat# 11595030 | |
| GraphPad Prism 8 | Graphpad | N/A | |
| HBSS | Thermo Fisher Scientific | Cat# 88284 | |
| HEPES | Sigma- Aldrich | Cat# H4034 | |
| jetPRIME Reagent | Polyplus Transfection | Cat# 114-15 | transfection reagent |
| Janamycin | Solarbio | Cat# K1030 | |
| LB medium | Invitrogen | Cat# 12780052 | |
| Leupeptin | Sigma-Aldrich | Cat# L2884 | |
| LMNG | Anatrace | Cat# NG310 | |
| MotionCor2 | (Zheng et al., 2017) | https://emcore.ucsf.edu/ucsf-software | |
| NanoCab | Thermo Fisher Scientific | Cat# 1121822 | |
| PBS | Invitrogen | Cat# 14190-144 | |
| pcDNA3.1-HA-FLAG-S1PRs | GenScript | N/A | |
| pFastBac1-Gαi | GenScript | N/A | |
| pFastBac1-HA-FLAG-T4L-S1PRs-His10 | GenScript | N/A | |
| pFastBacdual-Gβ1γ2 | GenScript | N/A | |
| PureLink HiPure Plasmid Miniprep Kit | Invitrogen | Cat# K210003 | For the preparation of plasmids and P0 baculovirus |
| Q5 site-Directed Mutagenesis kit | NEB | Cat# E0554S | For the preparation of plasmids |
| Quantifoil | Quantifoil | Cat# 251448 | |
| RELION-3.1 | (Zivanov et al., 2018) | https://www2.mrc-lmb.cam.ac.uk/relion | |
| S1PRs cDNA | addgene | N/A | |
| scFv16 | Invitrogen | Cat# 703976 | |
| Sf9 | Expression Systems | N/A | |
| Siponimod | Selleck | Cat# S7179 | |
| sodium cholate | Sigma-Aldrich | Cat# C1254 | |
| Synergy H1 microplate reader | BIO-TEK | N/A | |
| Synthetic T4L DNA (sequence) | N/A | N/A | Aacatcttcgagatgctgcgcatcgacgaagg cctgcgtctcaagatttacaagaataccgaagg ttattacacgattggcatcggccacctcctgaca aagagcccatcactcaacgctgccaagtctga actggacaaagccattggtcgcaacaccaac ggtgtcattacaaaggacgaggcggagaaac tcttcaaccaagatgtagatgcggctgtccgtgg catcctgcgtaatgccaagttgaagcccgtgt atgactcccttgatgctgttcgccgtgcagcctt gatcaacatggttttccaaatgggtgagaccgg agtggctggttttacgaactccctgcgcatgctcc agcagaagcgctgggacgaggccgcagtga atttggctaaatctcgctggtacaatcagacacc taaccgtgccaagcgtgtcatcactaccttccg tactggaacttgggacgcttac |
| TCEP | Thermo Fisher Scientific | Cat# 77720 | |
| Tetracycline | Solarbio | Cat# T8180 | |
| Vitrobot Mark IV | Thermo Fisher Scientific | N/A |
References
- Verstockt, B., et al. Sphingosine 1-phosphate modulation and immune cell trafficking in inflammatory bowel disease. Nature Reviews: Gastroenterology & Hepatology. , 1-16 (2022).
- Rosen, H., Stevens, R. C., Hanson, M., Roberts, E., Oldstone, M. B. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annual Review of Biochemistry. 82, 637-662 (2013).
- Cartier, A., Hla, T. Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science. 366 (6463), 5551 (2019).
- Jozefczuk, E., Guzik, T. J., Siedlinski, M. Significance of sphingosine-1-phosphate in cardiovascular physiology and pathology. Pharmacological Research. 156, 104793 (2020).
- Kihara, Y., Maceyka, M., Spiegel, S., Chun, J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. British Journal of Pharmacology. 171 (15), 3575-3594 (2014).
- Bryan, A. M., Del Poeta, M. Sphingosine-1-phosphate receptors and innate immunity. Cellular Microbiology. 20 (5), 12836 (2018).
- Pelletier, D., Hafler, D. A. Fingolimod for multiple sclerosis. New England Journal of Medicine. 366 (4), 339-347 (2012).
- Obinata, H., Hla, T. Sphingosine 1-phosphate and inflammation. International Immunology. 31 (9), 617-625 (2019).
- Pyne, N. J., Pyne, S. Sphingosine 1-phosphate and cancer. Nature Reviews: Cancer. 10 (7), 489-503 (2010).
- Abu-Farha, M., et al. The role of lipid metabolism in COVID-19 virus infection and as a drug target. International Journal of Molecular Sciences. 21 (10), 3544 (2020).
- Chun, J., Kihara, Y., Jonnalagadda, D., Blaho, V. A. Fingolimod: lessons learned and new opportunities for treating Multiple Sclerosis and other disorders. Annual Review of Pharmacology and Toxicology. 59, 149-170 (2019).
- Murakami, A., et al. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: investigation based on a new S1P3 receptor antagonist. Molecular Pharmacology. 77 (4), 704-713 (2010).
- Cao, L., et al. Siponimod for multiple sclerosis. Cochrane Database of Systematic Reviews. 11, (2021).
- Scott, L. J. Siponimod: a review in secondary progressive Multiple Sclerosis. CNS Drugs. 34 (11), 1191-1200 (2020).
- Lamb, Y. N. Ozanimod: first approval. Drugs. 80 (8), 841-848 (2020).
- Scott, F. L., et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. British Journal of Pharmacology. 173 (11), 1778-1792 (2016).
- McGowan, E. M., Haddadi, N., Nassif, N. T., Lin, Y. Targeting the SphK-S1P-SIPR pathway as a potential therapeutic approach for COVID-19. International Journal of Molecular Sciences. 21 (19), 7189 (2020).
- O'Sullivan, C., Dev, K. K. The structure and function of the S1P1 receptor. Trends in Pharmacological Sciences. 34 (7), 401-412 (2013).
- Liao, M., Cao, E., Julius, D., Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 504 (7478), 107-112 (2013).
- Bai, X. C., McMullan, G., Scheres, S. H. How cryo-EM is revolutionizing structural biology. Trends in Biochemical Sciences. 40 (1), 49-57 (2015).
- Murata, K., Wolf, M. Cryo-electron microscopy for structural analysis of dynamic biological macromolecules. Biochimica et Biophysica Acta General Subjects. 1862 (2), 324-334 (2018).
- Zhang, M., et al. Cryo-EM structure of an activated GPCR-G protein complex in lipid nanodiscs. Nature Structural & Molecular Biology. 28 (3), 258-267 (2021).
- Renaud, J. P., et al. Cryo-EM in drug discovery: achievements, limitations and prospects. Nature Reviews: Drug Discovery. 17 (7), 471-492 (2018).
- Ishchenko, A., Gati, C., Cherezov, V. Structural biology of G protein-coupled receptors: new opportunities from XFELs and cryoEM. Current Opinion in Structural Biology. 51, 44-52 (2018).
- Yang, D., et al. G protein-coupled receptors: structure- and function-based drug discovery. Signal Transduction and Targeted Therapy. 6 (1), 7 (2021).
- Yuan, Y., et al. Structures of signaling complexes of lipid receptors S1PR1 and S1PR5 reveal mechanisms of activation and drug recognition. Cell Research. 31 (12), 1263-1274 (2021).
- Zhao, C., et al. Structural insights into sphingosine-1-phosphate recognition and ligand selectivity of S1PR3-Gi signaling complexes. Cell Research. 32 (2), 218-221 (2022).
- Xu, Z., et al. Structural basis of sphingosine-1-phosphate receptor 1 activation and biased agonism. Nature Chemical Biology. 18, 281-288 (2022).
- Liu, Y. F., Ghahremani, M. H., Rasenick, M. M., Jakobs, K. H., Albert, P. R. Stimulation of cAMP synthesis by Gi-coupled receptors upon ablation of distinct Galphai protein expression. Gi subtype specificity of the 5-HT1A receptor. Journal of Biological Chemistry. 274 (23), 16444-16450 (1999).
- Buccioni, M., et al. Innovative functional cAMP assay for studying G protein-coupled receptors: application to the pharmacological characterization of GPR17. Purinergic Signalling. 7 (4), 463-468 (2011).
- Wang, F. I., Ding, G., Ng, G. S., Dixon, S. J., Chidiac, P. Luciferase-based GloSensor cAMP assay: Temperature optimization and application to cell-based kinetic studies. Methods. , (2021).
- Audet, M., et al. Small-scale approach for precrystallization screening in GPCR X-ray crystallography. Nature Protocols. 15 (1), 144-160 (2020).
- Sgro, G. G., Costa, T. R. D. Cryo-EM grid preparation of membrane protein samples for single particle analysis. Frontiers in Molecular Biosciences. 5, 74 (2018).
- White, J. B. R., et al. Single particle cryo-electron microscopy: from sample to structure. Journal of Visualized Experiments. (171), e62415 (2021).
- Thompson, R. F., Iadanza, M. G., Hesketh, E. L., Rawson, S., Ranson, N. A. Collection, pre-processing and on-the-fly analysis of data for high-resolution, single-particle cryo-electron microscopy. Nature Protocols. 14 (1), 100-118 (2019).
- Fernandez-Leiro, R., Scheres, S. H. W. A pipeline approach to single-particle processing in RELION. Acta Crystallographica Section D. 73 (6), 496-502 (2017).
- Punjani, A., Rubinstein, J. L., Fleet, D. J., Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nature Methods. 14 (3), 290-296 (2017).
- Brilot, A. F., et al. Beam-induced motion of vitrified specimen on holey carbon film. Journal of Structural Biology. 177 (3), 630-637 (2012).
- Zheng, S. Q., et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nature Methods. 14 (4), 331-332 (2017).
- Zhang, K. Gctf: Real-time CTF determination and correction. Journal of Structural Biology. 193 (1), 1-12 (2016).
- Scheres, S. H. Semi-automated selection of cryo-EM particles in RELION-1.3. Journal of Structural Biology. 189 (2), 114-122 (2015).
- Liu, S., et al. Differential activation mechanisms of lipid GPCRs by lysophosphatidic acid and sphingosine 1-phosphate. Nature Communications. 13 (1), 731 (2022).
- Duan, J., et al. Cryo-EM structure of an activated VIP1 receptor-G protein complex revealed by a NanoBiT tethering strategy. Nature Communications. 11 (1), 4121 (2020).
- DiIorio, M. C., Kulczyk, A. W. A robust single-particle cryo-electron microscopy (cryo-EM) processing workflow with cryoSPARC, RELION, and Scipion. Journal of Visualized Experiments. (179), e63387 (2022).
- Pradelles, P., Grassi, J., Chabardes, D., Guiso, N. Enzyme immunoassays of adenosine cyclic 3',5'-monophosphate and guanosine cyclic 3',5'-monophosphate using acetylcholinesterase. Analytical Chemistry. 61 (5), 447-453 (1989).
- Jiang, L. I., et al. Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. Journal of Biological Chemistry. 282 (14), 10576-10584 (2007).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved