Drug Treatment by Central Venous Catheter in a Mouse Model of Angiotensin II Induced Abdominal Aortic Aneurysm and Monitoring by 3D Ultrasound
In This Article
Summary
This protocol describes the consecutive implantation of an osmotic pump to induce abdominal aortic aneurysm by angiotensin II release in apolipoprotein E (ApoE) deficient mice and of a vascular access port with a jugular vein catheter for repeated drug treatment. Monitoring of aneurysm development by 3D ultrasound is effectively conducted despite dorsal implants.
Abstract
Since pharmaceutical treatment options are lacking in the clinical management of abdominal aortic aneurysm (AAA), animal models, in particular mouse models, are applied to advance the understanding of the disease pathogenesis and to identify potential therapeutic targets. Testing novel drug candidates to block AAA growth in these models generally requires repeated drug administration during the time course of the experiment. Here, we describe a compiled protocol for AAA induction, insertion of an intravenous catheter to facilitate prolonged therapy, and serial AAA monitoring by 3D ultrasound. Aneurysms are induced in apolipoprotein E (ApoE) deficient mice by angiotensin II release over 28 days from osmotic mini-pumps implanted subcutaneously into the mouse back. Subsequently, the surgical procedure for external jugular vein catheterization is conducted to allow for daily intravenous drug treatment or repeated blood sampling via a subcutaneous vascular access button. Despite the two dorsal implants, the monitoring of AAA development is readily facilitated by sequential semi-automated 3D ultrasound analysis, which yields comprehensive information on the expansion of aortic diameter and volume and on aneurysm morphology, as illustrated by experimental examples.
Introduction
An abdominal aortic aneurysm (AAA) is a pathological dilatation of a vessel due to inflammatory and tissue-destructive processes in the aortic wall that may ultimately lead to rupture and patient death. Despite considerable achievements in surgical AAA repair, a conservative drug treatment to block the progression of aneurysm expansion and potentially lower the risk of rupture is missing to date. Animal models have been developed to elucidate triggers and mediators of the disease and to test novel approaches to therapy. Mouse models of AAA are widely applied and cover the different observations from human tissue. Due to their pathomechanistic differences, often more than one model is applied to investigate the particular function of molecules/pathways or the efficacy of potential therapeutic drugs1,2. Among the most commonly used models of AAA induction is angiotensin-II (Ang-II) administration in apolipoprotein E deficient (ApoE KO) mice3, which has more chronic-like pathogenesis in comparison to models that rely on aneurysm formation from an acute insult to the aortic wall4,5. Thus, the Ang-II model seems particularly suited for monitoring disease progression and was recently shown to closely resemble human AAA disease in regard to metabolic and inflammatory responses6. Notably, the Ang-II model features not only AAA development but also thoracic aneurysm formation, as well as aortic dissection with intramural thrombus formation.
Treatments aimed at targeting the progression of already established AAA rather than preventing the initiation of the disease may have higher translational value as patients present with a pre-existing condition that requires treatment7,8. For a comparable experimental design, aortic size needs to be monitored before and after AAA induction to define a threshold of disease development and potentially stratify mice into treatment groups.
The mode of drug administration depends on the uptake and stability of the respective substance. Intraperitoneal (i.p.) injections are most often utilized due to their ease of application, not requiring anesthetic, and the lack of injection volume constraints9. Pharmacokinetics have to be considered, however, when choosing the route of administration, as substances administered i.p. are primarily absorbed through the hepatic portal circulation and may undergo liver metabolism before reaching circulation, which could result in varying plasma concentrations depending on the first pass effect10. Intravenous (i.v.) injection yields the highest bioavailability of substances, and the challenge of repetitive i.v. access can be circumvented by the use of catheters and vascular access ports for daily administration11,12,13. With respect to the AAA setting, drug distribution in circulation facilitates direct aneurysm exposure at defined concentrations.
Here, we describe a workflow for inducing AAA in the Ang-II mouse model via the subcutaneous implantation of an osmotic pump, for daily i.v. drug treatment via a vascular access port connected to a catheter inserted in the external jugular vein, as well as for the monitoring of aneurysm size via 3D ultrasound14 despite the presence of two dorsal implants.
Protocol
Animal experiments were approved by the local ethics committee and the Austrian Ministry of Science (BMWFW-66.009/0355-WF/V/3b/2016), conforming to the European Directive 2010/63/EU on the protection of animals used for scientific purposes and the Austrian Animal Experiment Act 2012. Humane endpoints were set as follows: loss of ≥15% body weight, avoiding food and/or water intake, reduced activity (hypokinesia) or dyskinesia, or prolonged shaking, scratching, labored respiration, or hunched posture despite pain/symptom management. If necessary, an animal is euthanized under deep anesthesia, i.e., an overdose cocktail of ketamine (approx. 100 mg/kg) and xylazine (approx. 5 mg/kg), or by cervical dislocation. For surgical procedures, aseptic technique and sterile/clean gloves are used throughout.
1. Pump implantation
- Anesthesia
- Keep ApoE deficient mice (B6.129P2-Apoetm1Unc/J) on a normal diet and preferably include 12-14 week old male animals in the experiments to represent the male predominance in human disease3.
- 1 day before surgery (d-1, pre-OP), prepare and fill the osmotic pumps with the desired concentration of angiotensin II according to mouse weight following the manufacturer's protocol and incubate the pumps in saline at 37 °C overnight15.
Example: For a 25 g mouse, using osmotic pumps (see Table of Materials) with a 1000 ng/kg/min delivery rate and a 0.25 µL/h pump rate for 28 days, dissolve 1.8 mg of Ang-II in 300 µL of saline (6000 ng/µL concentration for delivering 1500 ng/h of Ang-II). Load the solution with the blunt filling needle into the pump and then insert the flow moderator to close the pump. - Place the mouse in the anesthesia chamber at 3%-4% isoflurane mixed with 2 L/min O2 until unconscious. Move the mouse to a heated table (37 °C) in a prone position and maintain isoflurane anesthesia at 1.8%-2% through a nose cone.
- Apply eye lubricant to both eyes to prevent dryness.
- Inject the mouse with 2.5% buprenorphine in saline at 10 µL/g of mouse subcutaneously and verify the depth of anesthesia by a toe pinch.
- Shave a small area on the upper left side of the mouse back over the shoulder blade. Apply 10% (w/v) povidone-iodine solution for disinfection of the shaved area.
- Pump insertion (5-7 min, performed without a microscope)
- Check that the mouse is completely anesthetized by toe pinch and make a 1 cm transversal incision in the skin of the upper back with a scalpel between the midspinal and left scapular line.
- Hold the skin up with forceps and use blunt, curved scissors to make a subcutaneous pocket by pushing toward the left hind limb. Open the scissors, pull the opened scissors out of the cut and repeat to widen the pocket.
- Gently insert the pump into the pocket with the flow moderator toward the tail (to minimize potential interference of Ang-II release by the incision site).
NOTE: The pocket should not only be wide enough for the pump insertion but also for the skin to not be tight around the pump, and there should be at least 5 mm between the pump and incision site to allow for optimal wound healing. - Close the wound with 4-0 absorbable interrupted sutures.
- Inject the mouse with 10% glucose in saline at 10 µL/g of mouse subcutaneously.
- Apply povidone-iodine wound spray to the closed wound and allow the mouse to recover consciousness under a heating lamp, then return it to the cage with 7.5 mg of piritramide (for extended pain management) and 20 mL of 5% glucose in 200 mL of drinking water for 3 days post operation.
- Check on the mice several times per day for signs of pain or distress.
NOTE: Since aortic ruptures occur at a 20%-40% rate and predominantly within the first 3-10 days post operation, the risk of prolonged severe pain or distress needs to be minimized by frequent animal monitoring. The main indications for imminent rupture include: separation from the group, hunched posture, decreased mobility (to the extent of hind limb paralysis), and decreased or non-responsiveness during handling.
2. Jugular vein catheterization
NOTE: This surgical procedure requires a microscope with 8x-10x magnification.
- Using the vascular access system (see Table of Materials), prepare the catheter by cutting the 3Fr side to the desired length (~5-7 mm before the silicone anchor) and pushing the catheter over the 22 G metal connector of the vascular access system (VAS) with at least 3 mm overlap. Place the aluminum cap on the button to protect the port.
- Prepare 1-1.5 cm long 6-0 silk ligatures.
- Place the mouse in the anesthesia chamber at 3%-4% isoflurane mixed with 2 L/min O2 until unconscious.
- Move the mouse to a heated table (37 °C) in a supine position and maintain isoflurane anesthesia at 1.8%-2% through a nose cone.
- Apply eye lubricant to both eyes to prevent dryness.
- Inject the mouse with 2.5% buprenorphine in saline at 10 µL/g of mouse subcutaneously.
- Shave the fur from the right side of the neck on the ventral side and on the right side of the upper back (the left side will have the implanted osmotic pump).
- Apply the povidone-iodine solution for disinfection of the shaved area.
- Check that the mouse is completely anesthetized by toe pinch.
- Jugular vein preparation (5-10 min, performed under the microscope)
- Make a 0.5 cm transversal supraclavicular skin incision at the right side of the neck over the right clavicle.
- Use blunt microsurgical tweezers to separate the connective tissue and fat, exposing the external jugular vein. Avoid tearing apart small blood vessels in the fat.
- Isolate at least 5 mm of the vessel, close to the pectoral muscles.
- Blunt dissect tissue under the vein using bent micro tweezers and pass through 2-3 of the 6-0 ligatures.
NOTE: If any side branches are identified in the area of interest, either the ligature should be inserted to be caudal to the side branch or the side branch should be permanently ligated by isolating and tying off with a 6-0 ligature. - Tuck in the ligatures and add a drop of saline to the site.
- Button implantation (5-7 min, performed without a microscope)
- Flip the mouse over and place it in the prone position; verify the depth of anesthesia by a toe pinch and apply the povidone-iodine solution to disinfect the shaved area.
- Make a 1 cm sagittal incision on the upper back with a scalpel between the midspinal and right scapular lines.
- Use blunt curved scissors to make a circular pocket only slightly larger than the size of the VAS around the incision site by blunt dissection.
- Use the blunt curved scissors to tunnel cranially over the right shoulder toward the ventral incision at the neck by slightly opening the scissors, then pulling the opened scissors out, and repeating the action as it is pushed further in.
NOTE: The mouse can be turned on its left side for this step. - Once the tunnel has reached the ventral incision, pass through surgical clamps from the ventral to the dorsal incision.
- Attach the 3Fr end of the catheter to the clamp and pull the catheter through the tunnel so that it is out of the ventral neck incision and the VAS is in place at the dorsal incision.
- Insert the VAS's surgical felt disk subcutaneously at the incision at the back.
- Unclamp the catheter and flush with saline or phosphate-buffered saline without calcium and magnesium (PBS-/-), check for patency by using the fork end of the handling tool to remove the protective aluminum cap, and then use the magnetic end to hold the button and inject with a 1 mL syringe attached to the corresponding injector until the liquid leaks from the 1Fr end.
NOTE: Catheter flushing may alternatively be conducted in step 2.1. - Push the button caudally in the pocket and close the skin over the felt disk of the VAS, under the flange of the VAS, with at least two 4-0 interrupted sutures cranially.
NOTE: Ensure no tension on the skin around the button.
- Vein catheterization (7-10 min, performed under the microscope)
- Flip the mouse back to the supine position, verify the depth of anesthesia by a toe pinch, and add a drop of saline to the cut site.
- Tie the first ligature around the catheter and the jugular vein with 2-3 knots as far cranially as possible to ligate the vein and anchor the catheter to the outside. Move the second ligature as close as possible to the pectoral muscles.
- Shorten the catheter to the required length so that ~3-5 mm of the catheter is in the vein by cutting with micro-scissors at a diagonal angle to create a sharp end.
- Pierce a hole in the vein using a 27 G needle attached to a 1 mL syringe filled with saline by pulling on the secured cranial ligature and pushing the needle parallel to the vein.
NOTE: If blood from backflow leaks from the vein, use a cotton swab to apply pressure until the bleeding stops. - Insert the catheter into the vein in the same manner by pulling on the secured cranial ligature and sliding the catheter into the vein using the bent tweezers. Push the catheter until it is aligned with the vein.
- Tie the second ligature over the region where the catheter is inserted into the vein with 2-3 knots and check that there is no blood leakage. A third ligature and some of the local fat tissue may be used to additionally secure the catheter.
- Cut off the excess end of both ligatures with micro-scissors and add a drop of saline.
- Close the skin with 4-0 absorbable interrupted sutures.
- Inject the mouse with 10% glucose in saline at 10 µL/g of mouse subcutaneously.
- Inject the mouse with the desired volume of inhibitor or PBS/saline by using the fork end of the handling tool to remove the protective aluminum cap and then the magnetic end to hold the button and inject with a 1 mL syringe attached to the injector.
NOTE: Make sure there is no air or air bubbles in the injection syringe by pressing the plunger until a drop of liquid comes out before injecting. Maintain positive pressure on the plunger while disconnecting the syringe with the injector from the VAS to prevent pulling blood into the catheter tip and causing catheter blockage. - Apply a povidone-iodine wound spray to the closed wound and allow the mouse to recover consciousness under a heating lamp, then return it to the cage with 7.5 mg of piritramide (for extended pain management) and 20 mL of 5% glucose in 200 mL of drinking water for 3 days post operation.
- Check on the mice several times per day for signs of pain or distress.
- Daily injections (<5 min)
- For daily injection, place the mouse in the anesthesia chamber at 3%-4% isoflurane mixed with 2 L/min O2 until it is unconscious and its breathing rate is slowed down, then inject as in step 2.12.10. Check the neck for signs of swelling post-injection, which would indicate that the catheter is no longer inserted in the vein. Also, note that injection will not be possible if the catheter is occluded.
NOTE: A 10 µL/g of mouse weight 1x per day injection is well tolerated by the mice.
- For daily injection, place the mouse in the anesthesia chamber at 3%-4% isoflurane mixed with 2 L/min O2 until it is unconscious and its breathing rate is slowed down, then inject as in step 2.12.10. Check the neck for signs of swelling post-injection, which would indicate that the catheter is no longer inserted in the vein. Also, note that injection will not be possible if the catheter is occluded.
3. 3D ultrasound
- Prepare the ultrasound imaging system, heating table, and gel warmer, attach the transducer to the system, and set up above the stage in a transverse position (i.e., perpendicular to the mouse spine).
- Using the ultrasound software, adjust the settings to gain of 30 dB, image depth of 9.0 mm, and image width of 8.08 mm.
- Place the mouse in the anesthesia chamber at 3%-4% isoflurane mixed with 2 L/min O2 until unconscious. Move the mouse to a heated table (37 °C) in the supine position and maintain isoflurane anesthesia at 1.8%-2% through a nose cone.
- Apply eye lubricant to both eyes to prevent dryness.
- Shave the fur on the mouse abdomen. Apply hair removal cream for 1 min if needed, then wipe off and clean with damp gauze.
- Add a drop of electrode gel to each of the four electrocardiogram (ECG) electrodes on the stage and tape the mouse extremities to them.
- Spread warm ultrasound gel on the mouse's abdomen and lower the transmitter to set it in contact with the animal.
- Identify the aorta as a circular fast pulsating vessel.
NOTE: The inferior vena cava (IVC) will be located next to the aorta, and if the probe is pressed down firmly, the IVC will be compressed while the aorta remains stable. Confirmation that the analyzed vessel is the aorta rather than the IVC can be obtained using the pulse wave Doppler (PW-mode), with an angle of 65°. The aorta will have a high pulse wave velocity. - Locate the left renal artery and survey the area manually up to 12 mm cranially to ensure there is no interference in the area of interest (i.e., suprarenal aorta). Return to the left renal artery then set the probe at 6 mm cranially from the left renal artery.
NOTE: The 3D ultrasound will record the specified length (i.e., 12 mm) starting halfway (6 mm) caudally from the point of origin and up the specified length (12 mm) cranially. Troubleshooting steps for interference include pressing down slightly with the transducer, lifting then lowering the transducer again, applying more ultrasound gel, and tilting the angle of the stage. - 3D ultrasound acquisition
- Set the respiratory gating to 25% delay and a window of 50% and the ECG trigger (T1) to 50 ms (to record peak systolic dilation of the aorta).
NOTE: Respiratory gating can be optimized for each animal based on the respiratory rate and effort to ensure movement artifacts are removed. - From the 3D options, set the scan distance to 11.96 mm with a step size of 0.076 mm, which results in 157 frames.
- The program will automatically acquire the 157 frames in approximately 1-2 min. Scroll through to check for image quality, repeat if subpar, then save the image.
- Set the respiratory gating to 25% delay and a window of 50% and the ECG trigger (T1) to 50 ms (to record peak systolic dilation of the aorta).
- 2D diameter acquisition
- Turn off the respiratory gating and ECG trigger, and manually locate the area with the largest diameter in the 12 mm stretch of the suprarenal aorta.
- Acquire a B-mode image16.
- Additionally, without moving the transducer, acquire an ECG-gated kilohertz visualization (EKV) image with the system's standard settings at the same site.
- Ending the scan
- Wipe the ultrasound gel from the abdomen and return the mouse to its cage. Monitor the mouse until it fully recovers.
4. Ultrasound analysis
- Volume analysis
- In the analysis software, open the 3D Mode image, and under the Image Processing menu, press on Load into 3D, which will compile the 157 2D frames into a 3D image (i.e., cube).
- In the Volume Measurement menu, choose Parallel & Rotational Methods, and then the software will display the 3D image in a single pane.
- Under Volume, press Start, and draw the first contour around the aorta's inner wall by clicking to add the first point, moving the cursor around the aorta, and then right-clicking to complete the contour.
- Skip 9-10 frames (0.75-1 mm), then draw another contour in the same manner. Repeat these steps until the last frame is reached. This should result in 16-17 contours.
NOTE: The first and last frames have to have contours drawn in order for the correct volume over 12 mm to be calculated. - Select the first contour from the menu and choose Refine. This will initiate the edge detection algorithm to closely fit the line to the vessel wall. Move the points on the contour by dragging them to a new position so that the contour accurately lines the aorta's inner wall edge.
NOTE: In the Ang-II model, an intramural thrombus might be present. Since this is a common feature of this model, volume measurement should include the thrombus. - Refine all the contours and press Finish to save the analysis. The calculated volume will be displayed in the bottom left corner.
- Diameter analysis
NOTE: Diameter measurements may be conducted inner-to-inner wall, outer-to-outer wall, or inner-to-outer wall but must be consistent for all measurements. However, in the Ang-II model, an intramural thrombus might be present, which should be included in the analysis.- From the 3D mode image: Evaluate the 157 frames to identify the maximum diameter by visual inspection. Then, from the Measurement menu, choose Linear and draw multiple lines across the aorta to determine the largest diameter.
- From the B-mode or EKV (ECG-gated kilohertz visualization) image: In the cine loop, identify the maximum expansion of the aorta (at systole) by visual inspection. Then, from the Measurement menu, choose Linear and draw multiple lines across the aorta to determine the largest diameter.
NOTE: The ECG can be used to determine the cardiac cycle, but visual identification yields accurate results.
Representative Results
Representative results show the development and progression of the suprarenal aneurysms as monitored by ultrasound at baseline, day 8, and day 27 (Figure 1A). A trichrome stain (Figure 1B) of the day 27 aorta in Figure 1A further illustrates the morphology of the formed aneurysm with wall dissection and intramural thrombus. Aortic volume (mm3) was determined over a stretch of 12 mm14, and maximum aortic diameter was additionally measured from the EKV images. A threshold of 125% volume growth from baseline to day 8 was set for defining initial aneurysm development. Based on data collected over 2 years (2020-2021, n = 157), only 9% of animals failed to form an AAA according to this cutoff. However, 35% of the mice experienced aortic ruptures (thoracic or abdominal) prior to catheter implantation on day 9, thus resulting in a total of 56% of the remaining animals with established AAA disease amenable to stratification into treatment groups (Figure 1C). Of note, among our historic PBS controls (n = 21), aneurysms developed to varying degrees (range: 128%-314%, mean 199% ± 55% SD aortic volume growth at day 8). Importantly, an inverse relation was observed between the initial expansion and further disease progression, i.e., 55% of fast progressing aneurysms (>200% volume growth at day 8) did not progress further until day 27, while 80% of the other aneurysms (>125% and <200% volume growth at day 8) continued to expand until the end of the experiment (Figure 1D).
As recently reported14,17, the described methods have been successfully established, validated, and implemented, e.g., to document the therapeutic effect of a histone citrullination inhibitor (GSK484, for the inhibition of neutrophil extracellular trap formation) in blocking the progression of established AAA. ApoE deficient mice received Ang-II at 1000 ng/kg/min by subcutaneously implanted osmotic pumps over 28 days. Animals were stratified 1:1 to GSK484 (0.2 µg/g/day) or PBS treatment based on the aortic volume measured on day 8 and underwent the jugular vein catheterization procedure on day 9. Drug injections were conducted daily in a volume of 10 µL/g of mouse weight until the end of the study17. Figure 2 shows exemplary (n = 2/group) ultrasound results (time course of absolute and relative volume or diameter expansion), revealing that GSK484 treatment inhibited AAA progression, while the aneurysms continued to enlarge in control mice.
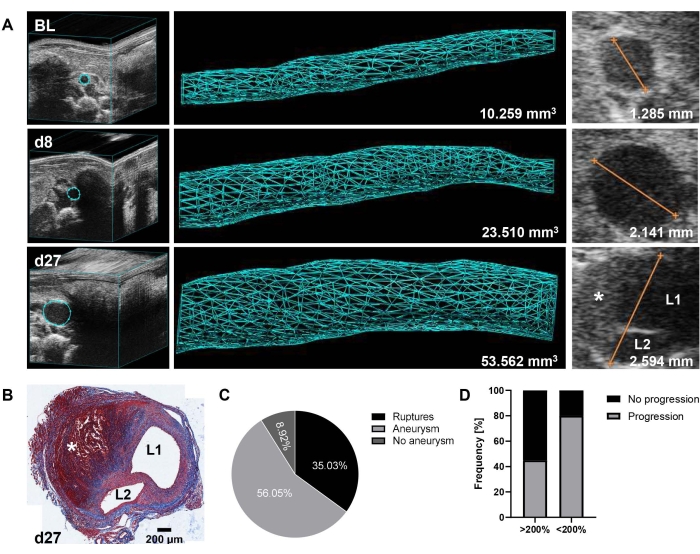
Figure 1: AAA formation and progression in the Ang-II mouse model as detected by 3D ultrasound. (A) The suprarenal aorta was monitored by 3D ultrasound at baseline (BL), day 8 (d8), and day 27 (d27) after Ang-II pump implantation. Volume was measured over a 12 mm stretch of the suprarenal aorta (157 frames) based on a 3D reconstructed image. The maximum aortic diameter was determined from EKV images. (B) Trichrome stain of a transverse section of the day 27 aorta after mouse sacrifice and organ collection. The presence of an aorta dissection is indicated by L1/L2 (lumen 1 and lumen 2), and the intramural thrombus is denoted by * in A and B. (C) Incidence rate of AAA (>125% aortic volume growth from BL) at day 8 and aortic ruptures within the first 9 days (thoracic or abdominal) from a data set collected over 2 years (n = 157). (D) Progression frequency from day 8 to day 27 of initially fast forming (>200% aortic volume growth from BL to day 8) versus moderately growing (>125% and <200% aortic volume growth from BL to day 8) aneurysms in PBS control-treated mice (n = 21). Please click here to view a larger version of this figure.
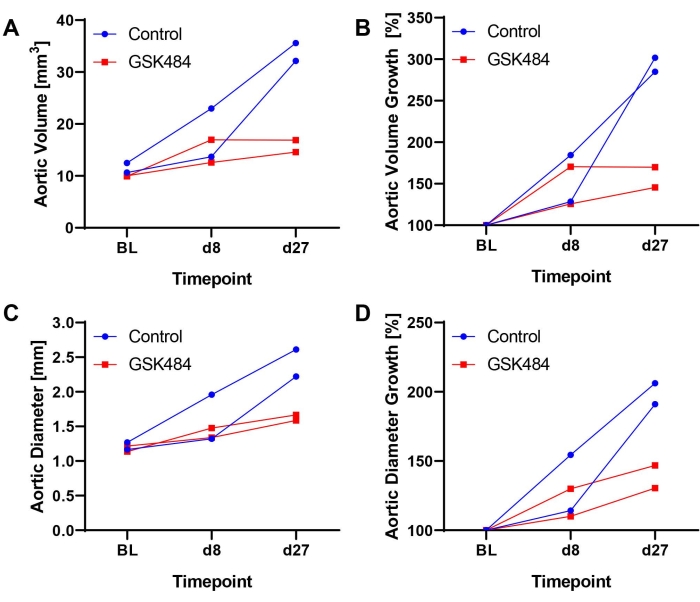
Figure 2: Exemplary results from inhibition of histone citrullination to block AAA progression in the Ang-II model by intravenous injection of GSK484 or PBS via vascular access button. (A) Aortic volume (mm3) as measured over a 12 mm stretch of the suprarenal aorta. (B) Calculated aortic volume growth from baseline (BL = 100%). (C) Maximum aortic diameter as determined from EKV images. (D) Calculated aortic diameter growth from BL. GSK484 data were extracted from a previously published study17. Please click here to view a larger version of this figure.
Discussion
The Ang-II model is one of the most commonly used mouse models of AAA due to its low technical demands and particular features resembling human disease3,6. The surgery time is about 10 min per animal, and the subcutaneous pump implantation is well tolerated by the mice if the subcutaneous pocket is sufficiently wide and placed low on the animal's back, away from the incision site, so as not to interfere with wound healing. When the skin is tight around the pump, tissue irritation may occur, which can cause inflammation and scabbing and potentially disrupt the pump's mechanism of release by osmotic pressure. Measuring the volume of Ang-II remaining in the pump at the time of animal sacrifice gives insight as to whether the Ang-II was successfully released over the 28 days.
The Ang-II model has recently been proposed to be well suited to study aortic aneurysm and dissection progression as it exhibits resemblance with human features of both6. Importantly, testing drug candidates to block aortic expansion and influence remodeling would match the current clinical demand. In our experimental setting, a cutoff for aneurysm formation was defined prior to the treatment start based on 125% volume growth on day 8 in relation to baseline, which accounts for the natural variation in absolute aorta size in mice. The threshold and time point were derived from an initial time course that confirmed aorta wall destruction in histology (data not shown) and resulted in 35% ruptures and 56% observed AAAs prior to catheter implantation. While a minimum threshold of established disease was applied for study inclusion, it was subsequently observed that a high extent of initial aorta expansion may also limit experimental applicability. Aneurysms that progressed rapidly to >200% volume by day 8 did not further grow beyond that size in 55% of cases (Figure 1D). This has to be taken into account during experimental design and sample size calculation, as it could mask a treatment's true effect. Another facet of this model is the frequent aortic ruptures (thoracic or abdominal), occurring at rates of 20%-40% and mostly within the first 10 days after Ang-II pump implantation3,18,19. Thus, by choosing the start of treatment to be day 9, a high rate of established aneurysms was achieved, and the jugular vein catheterization was essentially performed on mice that were expected to survive to the end of the experiment (only 3/24 mice in our historic control group ruptured post day 9), thus conserving time, effort, and cost.
Apart from the aorta ruptures, which constitute a severe condition, the concurrent implantation of the catheter with vascular access button and the osmotic pump was well tolerated by the mice, with no notable effect on mobility or behavior post recovery from surgery. The jugular vein catheterization procedure should take about 30 min for trained researchers. The duration of exposure to (isoflurane) anesthesia should be kept to a minimum, and the animal breathing rate has to be closely monitored to prevent breathing depression, which may lead to a fatal outcome if not resolved20. Blood loss after puncturing the jugular vein for catheter insertion - leading to animal death if major - could potentially occur when the jugular vein is not properly ligated cranially or a side branch feeding into the isolated area of the vessel is not closed off. In that case, pressure with a cotton swab should be applied to the puncture site until blood leakage slows or stops, then the catheter insertion and ligation should be done as quickly as possible; a small piece of the collagen wound dressing may be temporarily utilized to aid with hemostasis.
Catheter patency is one of the most important factors, as catheter disconnection from the vein or the access button results in improper drug delivery where the drug leaks into the subcutaneous space. Following the manufacturer's recommendation of a minimum of 3 mm overlap between the catheter and metal connecter, only one case of catheter disconnection at the button side (indicated by the injected liquid leaking from the incision site at the button) was recorded over 3 years in this model (2020-2021, n = 73), which was fixed by opening the wound and re-establishing the connection in surgery. In addition, a catheter patency failure rate of around 10% in our historic PBS control group (2/21) was experienced due to either catheter occlusion (making it impossible to inject), catheter disconnection from the vein (indicated by apparent swelling in the neck during injection), or wound healing complications. These issues may be connected to self-inflicted injuries, i.e., mouse scratches or bites. Notably, drug treatments that interfere with wound healing may raise failure rates. Troubleshooting steps to improve the patency rate include increasing the length of the catheter inserted in the vein, ensuring ligatures are tightly knotted around the catheter and vein, and applying the positive pressure technique following the manufacturer's recommendation, as described in step 2.12.10., while injecting. Catheter patency should, additionally, be verified at the time of animal sacrifice by dissection and visual inspection under the microscope. Of note, the daily volume of injected drug solution has to be carefully considered. As plasma volume regulates blood pressure, the injection volume may affect AAA expansion, and, hence, control animals need to receive the sham procedure with carrier volume. Based on our experience (and unpublished observations), a daily amount of up to 250 µL of PBS seems to be well tolerated. Finally, similar to the pump implantation, skin irritation can occur around the implanted vascular access button. If inflammation accompanied by devitalized or necrotic tissue is observed, wound debridement should be carried out by removing non-viable tissue (necrotic tissue will often separate naturally from the wound), and the skin should be sutured if needed; if inflammation and necrosis are extensive, the animal's welfare and humane endpoints have to be considered according to guidelines.
Single and dual dorsal implantation of the osmotic pump and/or the VAS did not interfere with the ultrasound signal nor with securing the mouse in an appropriate position on the ultrasound stage. The automated acquisition of 157 frames over 12 mm to render a 3D image of the aorta for volume measurement is a simple and fast procedure14, which only requires ensuring the aorta is clear of interference over the area of interest. One pitfall in this context is applying too much pressure with the transducer while attempting to clear the image of interference, which may interrupt the automated measurement if the breathing rate is affected by the compression of the ribs when images of the cranial end of the abdominal aorta are recorded. Diameter is traditionally measured in images acquired using B-mode by the operator manually searching for the area of maximum diameter while conducting the ultrasound analysis. An advancement on the B-mode images is the EKV images, which can resolve small aortic motions to produce a high-quality, slowed-down image of the pulsating aorta. Furthermore, the maximum aortic diameter can be determined from the acquired 3D frames, where the 157 images offer a comprehensive overview of the aorta taken at systole (due to the set ECG trigger).
In conclusion, the presented compiled protocol provides a reliable and reproducible workflow for i.v. drug administration in a mouse model of Ang-II induced AAA and for monitoring aortic size by 3D ultrasound. The time points of monitoring and operation can be adjusted to the specific needs, and the jugular vein catheterization can be performed separately for any experimental setup requiring delivery of specific substances via i.v. injections. The VAS can alternatively be used for repeated blood sampling if a catheter lock solution is used to prevent clotting. The described 3D ultrasound procedure may be adapted to measure the infrarenal aorta, where aneurysms develop upon acute insult in elastase or CaCl2-based mouse models of AAA. While 3D ultrasound acquisition holds the advantage of giving an overview of the affected aorta region and aneurysm morphology, the image acquisition is more time-consuming and, hence, might be more cost-intensive. Another limitation of the protocol that should be acknowledged is the need for the animals to be anesthetized briefly for intravenous injections, while intraperitoneal administration is generally performed on conscious mice.
Acknowledgements
We would like to thank Prof. Podesser's and Prof. Ellmeier's teams (Dept. of Biomedical Research and Core Facility for Laboratory Animal Breeding and Husbandry, Medical University of Vienna) for assistance in the animal experiments. The AAA trichrome staining was kindly performed by Monika Weiss and Prof. Peter Petzelbauer (Dept. of Dermatology, Medical University of Vienna). This work was supported by the Austrian Science Fund [SFB project F 5409-B21]. Marc Bailey is personally supported by the British Heart Foundation [FS/18/12/33270].
Materials
| Name | Company | Catalog Number | Comments |
| 4-0 Polysorb sutures | Covidien | GL-46-MG | Braided absorbable suture CV-23 Taper |
| 6-0 Silk sutures | Ethicon | 639H | PERMA-HAND Silk |
| ALZET 2004 osmotic pumps | DURECT Corp | 298 | Osmotic mini pumps |
| Angiotensin-II | Bachem | 4006473.0100 | Angiotensin II acetate |
| Aquasonic Clear Ultrasound Transmission Gel | Parker Labs | PUSG-0308 | Ultrasound gel |
| Betadona Wound Spray | Mundipharma | Wound disinfectant spray (povidone-iodine spray) | |
| Betaisodona Solution | Mundipharma | 15973 | Wound disinfectant solution (povidone-iodine solution) |
| Catheter for mouse femoral vein/artery | Instech Laboratories Inc | C10PU-MFV1301 | 1 to 3Fr, 10.5 cm, collar @1.2 cm. Fits 22 G |
| Hair removal cream | |||
| Handling tool | Instech Laboratories Inc | VABMG | Handling tool for magnetic mouse Vascular Access Buttons |
| HYLO NIGHT Eye Oinment | URSAPHARM | 538922 | Eye lubricant cream |
| Needles and syringes of various sizes | 1 mL and 5 mL syringes, 27 G and 30 G needles | ||
| Olympus SZ51 Stereo microscope | Olympus Corporation | Dissection and inspection microscope | |
| PinPort injectors | Instech Laboratories Inc | PNP3M-50 | Injector for vascular access button |
| Protective aluminum cap | Instech Laboratories Inc | VABM1C | Protective aluminum cap for magnetic 1 channel mouse VAB |
| Signa Electrode Ultrasound Gel | Parker Labs | PE-1560 | Electrode gel |
| Small electric shaver | |||
| Surigcal and microsurgical equipment | |||
| Suprasorb C | Lohmann & Rauscher | 20482 | Collagen wound dressing |
| Vascular access button (VAB) | Instech Laboratories Inc | VABM1B/22 | Vascular Access Button for mouse, magnetic, 1 channel 22 G, injector |
| Vevo 3100 Imaging System | FUJIFILM VisualSonics Inc | 51073-51 | Ultrasound system |
| Vevo Lab 5.6.1 software | FUJIFILM VisualSonics Inc | Ultrasound analysis software | |
| Vevo MX550D transducer | FUJIFILM VisualSonics Inc | Linear Array Transducer For Vevo 3100 system | |
| Vevo Mouse Handling Table | FUJIFILM VisualSonics Inc | 11436 | Mouse heating, mouse core temperature capture and ECG pads for physiological monitoring |
References
- Busch, A., et al. Translating mouse models of abdominal aortic aneurysm to the translational needs of vascular surgery. JVS-Vascular Science. 2, 219-234 (2021).
- Golledge, J., Krishna, S. M., Wang, Y. Mouse models for abdominal aortic aneurysm. British Journal of Pharmacology. 179 (5), 792-810 (2022).
- Daugherty, A., Manning, M. W., Cassis, L. A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. Journal of Clinical Investigation. 105 (11), 1605-1612 (2000).
- Bhamidipati, C. M., et al. Development of a novel murine model of aortic aneurysms using peri-adventitial elastase. Surgery. 152 (2), 238-246 (2012).
- Phillips, E. H., et al. Morphological and biomechanical differences in the elastase and AngII apoE -/- rodent models of abdominal aortic aneurysms. BioMed Research International. 2015, 413189 (2015).
- Gäbel, G., et al. Parallel murine and human aortic wall genomics reveals metabolic reprogramming as key driver of abdominal aortic aneurysm progression. Journal of the American Heart Association. 10 (17), 20231 (2021).
- Phie, J., Thanigaimani, S., Golledge, J. Systematic review and meta-analysis of interventions to slow progression of abdominal aortic aneurysm in mouse models. Arteriosclerosis, Thrombosis, and Vascular Biology. 41 (4), 1504-1517 (2021).
- Lu, H. S., Owens, A. P., Liu, B., Daugherty, A. Illuminating the importance of studying interventions on the propagation phase of experimental mouse abdominal aortic aneurysms. Arteriosclerosis, Thrombosis, and Vascular Biology. 41 (4), 1518-1520 (2021).
- Al Shoyaib, A., Archie, S. R., Karamyan, V. T. Intraperitoneal route of drug administration: Should it be used in experimental animal studies. Pharmaceutical Research. 37 (1), 1-17 (2020).
- Turner, P. V., Brabb, T., Pekow, C., Vasbinder, M. A. Administration of substances to laboratory animals: Routes of administration and factors to consider. Journal of the American Association for Laboratory Animal Science. 50 (5), 600-613 (2011).
- Derde, S., et al. Use of a central venous line for fluids, drugs and nutrient administration in a mouse model of critical illness. Journal of Visualized Experiments. (123), e55553 (2017).
- Lu, W., et al. Microsurgical skills of establishing permanent jugular vein cannulation in rats for serial blood sampling of orally administered drug. Journal of Visualized Experiments. (178), e63167 (2021).
- Jespersen, B., Knupp, L., Northcott, C. A. Femoral arterial and venous catheterization for blood sampling, drug administration and conscious blood pressure and heart rate measurements. Journal of Visualized Experiments. (59), e3496 (2012).
- Waduud, M. A., et al. High-frequency three-dimensional lumen volume ultrasound is a sensitive method to detect early aneurysmal change in elastase-induced murine abdominal aortic aneurysm. Aorta. 9 (6), 215-220 (2020).
- Lu, H., et al. Subcutaneous angiotensin II infusion using osmotic pumps induces aortic aneurysms in mice. Journal of Visualized Experiments. (103), e53191 (2015).
- Sawada, H., et al. Ultrasound imaging of the thoracic and abdominal aorta in mice to determine aneurysm dimensions. Journal of Visualized Experiments. (145), e59013 (2019).
- Eilenberg, W., et al. Histone citrullination as a novel biomarker and target to inhibit progression of abdominal aortic aneurysms. Translational Research. 233, 32-46 (2021).
- Saraff, K., Babamusta, F., Cassis, L. A., Daugherty, A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 23 (9), 1621-1626 (2003).
- Trachet, B., Fraga-Silva, R. A., Jacquet, P. A., Stergiopulos, N., Segers, P. Incidence, severity, mortality, and confounding factors for dissecting AAA detection in angiotensin II-infused mice: A meta-analysis. Cardiovascular Research. 108 (1), 159-170 (2015).
- Cesarovic, N., et al. Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Laboratory Animals. 44 (4), 329-336 (2010).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved