A subscription to JoVE is required to view this content. Sign in or start your free trial.
Creation of a Knee Joint-on-a-Chip for Modeling Joint Diseases and Testing Drugs
In This Article
Summary
We provide detailed methods for generating four types of tissues from human mesenchymal stem cells, which are used to recapitulate the cartilage, bone, fat pad, and synovium in the human knee joint. These four tissues are integrated into a customized bioreactor and connected through microfluidics, thus generating a knee joint-on-a-chip.
Abstract
The high prevalence of debilitating joint diseases like osteoarthritis (OA) poses a high socioeconomic burden. Currently, the available drugs that target joint disorders are mostly palliative. The unmet need for effective disease-modifying OA drugs (DMOADs) has been primarily caused by the absence of appropriate models for studying the disease mechanisms and testing potential DMOADs. Herein, we describe the establishment of a miniature synovial joint-mimicking microphysiological system (miniJoint) comprising adipose, fibrous, and osteochondral tissue components derived from human mesenchymal stem cells (MSCs). To obtain the three-dimensional (3D) microtissues, MSCs were encapsulated in photocrosslinkable methacrylated gelatin before or following differentiation. The cell-laden tissue constructs were then integrated into a 3D-printed bioreactor, forming the miniJoint. Separate flows of osteogenic, fibrogenic, and adipogenic media were introduced to maintain the respective tissue phenotypes. A commonly shared stream was perfused through the cartilage, synovial, and adipose tissues to enable tissue crosstalk. This flow pattern allows the induction of perturbations in one or more of the tissue components for mechanistic studies. Furthermore, potential DMOADs can be tested via either "systemic administration" through all the medium streams or "intraarticular administration" by adding the drugs to only the shared "synovial fluid"-simulating flow. Thus, the miniJoint can serve as a versatile in vitro platform for efficiently studying disease mechanisms and testing drugs in personalized medicine.
Introduction
Joint diseases like osteoarthritis (OA) are highly prevalent and debilitating and represent a leading cause of disability worldwide1. It is estimated that in the US alone, OA affects 27 million patients and occurs in 12.1% of adults aged 60 and above2. Unfortunately, most drugs currently used to manage joint diseases are palliative, and no effective disease-modifying OA drugs (DMOADs) are available3. This unmet medical need primarily stems from the absence of an effective model for studying the disease mechanisms and developing potential DMOADs. The conventional two-dimensional (2D) cell culture does not reflect the 3D nature of joint tissues, and the culture of tissue explants is often hindered by significant cell death and usually fails to replicate the dynamic tissue interconnections4. In addition, genetic and anatomical differences significantly reduce the physiological relevance of animal models4.
Organs-on-chips (OoCs), or microphysiological systems, are a promising research field at the interface of engineering, biology, and medicine. These in vitro platforms are minimal functional units that replicate defined healthy or pathological features of their in vivo counterparts5. Furthermore, these miniaturized systems can host diverse cells and matrices and simulate the biophysical and biochemical interactions between different tissues. Therefore, a microphysiological system that can faithfully recapitulate the native synovial joint promises to offer an effective platform for modeling joint diseases and developing potential DMOADs.
Human mesenchymal stem cells (MSCs) can be isolated from many tissues throughout the body and differentiated into osteogenic, chondrogenic, and adipogenic lineages6. MSCs have been successfully used to engineer various tissues, including bone, cartilage, and adipose tissue6, thus meaning they represent a promising cell source for engineering the tissue components of the knee joint. We recently developed a miniature joint-mimicking microphysiological system, named miniJoint, that comprises MSC-derived bone, cartilage, fibrous, and adipose tissues7. In particular, the novel design enables tissue crosstalk by microfluidic flow or permeation (Figure 1). Herein, we present the protocols for the fabrication of the chip components, the engineering of the tissue components, the culture of the engineered tissues in the chip, and the collection of tissues for downstream analyses.
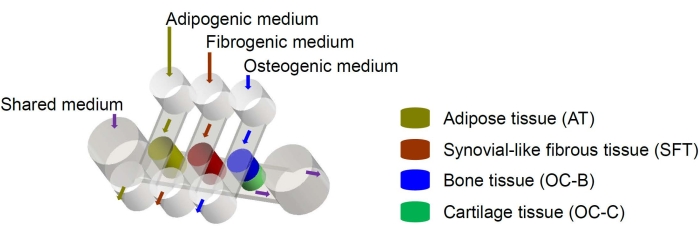
Figure 1: Schematic of the miniJoint chip showing the arrangement of the different tissue components and medium flows. OC = osteochondral tissue. Please click here to view a larger version of this figure.
Protocol
The following protocol follows the ethical guidelines of the University of Pittsburgh and the human research ethics committee of the University of Pittsburgh. Information on the materials used in this study is listed in the Table of Materials.
1. Manufacturing 3D-printed bioreactors
- Use a computer software to design osteochondral (Figure 2A) and miniJoint bioreactors (Figure 2B) that include chambers, inserts, and lids. The dimension information for each part is shown in Figure S1.
- Transfer the design to a 3D printer, and print with a photopolymer ink.
- Rinse the 3D-printed parts (Figure 2C-F) with 15 mL of 95% ethanol three times. Then, fully crosslink the printed pieces for 200 s in a flashlight polymerization device.
- Add O-rings to inserts and lids (Figure 2C, D), and test if the parts fit (Figure 2G, H).
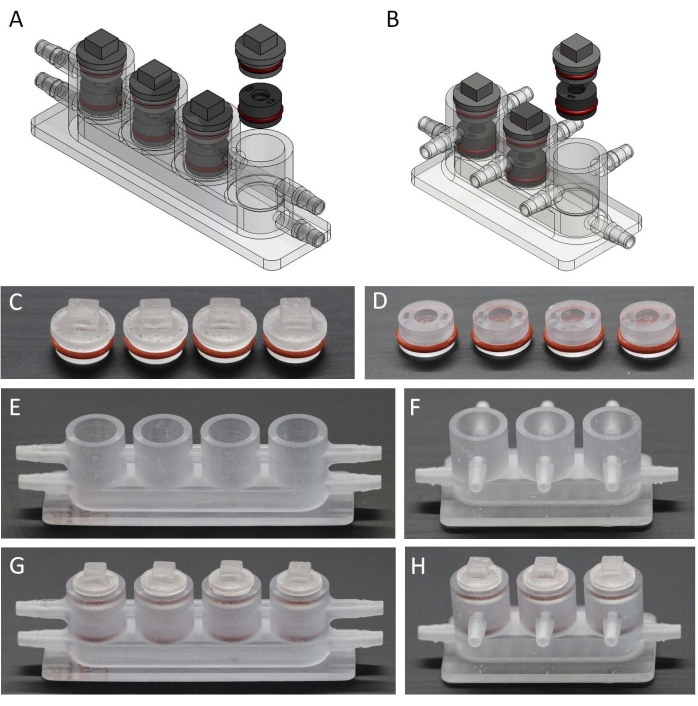
Figure 2: Fabrication of the different components to make the miniJoint bioreactor. (A,B), 3D models of bioreactors for creating (A) osteochondral and (B) miniJoint chips. (C,D) 3D printed (C) lids and (D) inserts with the O-ring installed. (E,F) 3D printed chambers for (E) osteochondral and (F) miniJoint tissue culture. (G,H) Assembly of (G) osteochondral and (H) miniJoint chips. Please click here to view a larger version of this figure.
2. Engineering the tissue components
NOTE: The processes for the fabrication of lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) and methacrylated gelatin (GelMA) are described in previous studies8,9.
- To create GelMA, follow the steps below.
- Add 17 g of gelatin Type B to 500 mL of distilled water, and then mix on a shaker for 30 min at 37 °C.
- Then, add 13 mL of methacrylic anhydride, place back on the 37 °C shaker, and allow to shake overnight.
- The following day, aliquot the GelMA into individual dialysis bags, with ~60 mL in each bag.
- Place all the dialysis bags in distilled water with a stir bar, and allow for 7 days of dialysis. Change the water multiple times per day, and leave the bags at 4 °C overnight.
- On day 7, freeze the GelMA at −80 °C. Once completely frozen, proceed to lyophilization.
- Place the GelMA in a dish in the vacuum chamber of a lyophilizer, and allow for freeze-drying. Ensure the GelMA is completely dried prior to removal from the lyophilizer.
- Dissolve the GelMA in Hank's balanced salt solution (HBSS with Ca2+ and Mg2+) at 15% (w/v). To ensure the pH is at 7.4, add NaOH in small amounts until the pH reaches 7.4. Supplement the solution with 1x antibiotic-antimycotic and 0.15% (w/v) LAP based on the volume acquired. Store the 15% GelMA solution at −20 °C until use, and protect it from light.
- Place 3D-printed dual-flow bioreactor chambers, lids, and inserts into autoclave bags, and autoclave at 121 °C for 20 min with steam and then for 20 min with dry heat.
- Inside the biological safety cabinet, soak the bioreactor chambers, lids, and inserts in 15 mL of sterile phosphate-buffered saline overnight, after which allow them to dry.
- Isolate human bone marrow-derived MSCs from total joint arthroplasty surgical waste with IRB approval (University of Pittsburgh and University of Washington).
- Specifically, flush out the bone marrow from the trabecular bone of the femoral neck and head, and resuspend it in Dulbecco's Modified Eagle's Medium (DMEM).
- Filter the suspension through a 40 µm strainer, and centrifuge the flow-through at 300 x g for 5 min.
- Remove the supernatant, resuspend the pellets using growth medium [DMEM, 10% fetal bovine serum (FBS), and 1x antibiotic-antimycotic], and then place into tissue culture flasks.
- Change the culture medium every 3 days to 4 days. Ensure that a confluence of 70% to 80% is reached before proceeding further.
- Detach the cells by incubating with trypsin-EDTA for 2-3 min, and passage at a ratio of 1 million cells per T150 flask.
- Expand the cells to P5. After trypsinization, suspend the cells, count them, and then pellet by centrifuging at 300x g for 5 min.
- With a 1,000 µL pipette, resuspend the cells at 20 x 106 cells/mL in 15% GelMA solution.
NOTE: Turn off the light of the biological safety cabinet. - Using sterile gloves, press a sterile, dry silicone mold against a Petri dish. Then, put one insert into each hole of the silicone mold with forceps, with the hole side of the insert facing down.
- Using a 200 µL pipette, add the cell suspension to fill up the insert (~50 µL per insert).
- Use a UV flashlight (LED light with a wavelength of 395 nm) to crosslink the top of the gel/insert for 1.5 min. Then, illuminate the other side for 30 s. Crosslinking occurs when the LAP photoinitiator is exposed to UV light.
NOTE: The cell suspension may be kept in the incubator during this period or protected from light. - With sterile forceps, immediately transfer the inserts into 8 mL of growth medium in a non-tissue culture 6-well plate (DMEM supplemented with 10% [v/v] FBS and 1x antibiotic-antimycotic) to allow the cells to recover overnight.
- Differentiate the cells toward four lineages.
- To engineer adipose tissue (AT), transfer the inserts to 8 mL of adipogenic medium (AM; Alpha-MEM, 10% FBS, 0.2 mM indomethacin, 1x insulin-transferrin-selenium (ITS), 0.45 mM 3-isobutyl-1-methylxanthine, 0.1 μM dexamethasone, and 1x antibiotic-antimycotic) to initiate differentiation. Ideally, four inserts are placed in a single well of a non-tissue culture well plate with 8 mL of adipogenic medium. Culture the cells in the well plates for 28 days, with medium changes every other day.
- To engineer the osteochondral units (OC), place the inserts into dual-flow bioreactor chambers, cap the wells, and infuse the two streams separately at a flow rate of 5 µL/min with 35 mL of osteogenic medium (OM; DMEM, 10% FBS, 1x antibiotic-antimycotic, 0.1 μM dexamethasone, 0.01 M β-glycerophosphate, 100 ng/mL bone morphogenic protein 7 (BMP7), 50 µg/mL ascorbic acid, and 10 nM vitamin D3) and 35 mL of chondrogenic medium (CM; DMEM, 1x antibiotic-antimycotic, 1x ITS, 0.1 μM dexamethasone, 40 µg/mL proline, 50 µg/mL ascorbic acid, and 10 ng/mL transforming growth factor β3)10. Maintain cell differentiation for 28 days by performing biweekly medium changes.
- To derive the fibroblasts, differentiate the MSCs in 2D culture over 21 days in a T150 cm2 tissue culture flask using 20 mL of fibrogenic medium (FM; advanced DMEM, 5% FBS, 1x GlutaMAX, 1x antibiotic-antimycotic, and 50 µg/mL ascorbic acid). Change the medium every week. Use 4 mL of trypsin to detach the cells, and encapsulate the 3D gels within the inserts, following the protocol described above, to obtain synovial like-fibrous tissue (SFT).
NOTE: The compositions of all the differentiation media can be found in Table 1.
3. Establishing the miniJoint chip
- Autoclave the 3D miniJoint bioreactor chambers, silicone tubing with a 0.062 inch inner diameter and a 0.125 inch outer diameter, and F 1/16 Luer-lock connectors. Connect the silicone tubing to the miniJoint bioreactor barb at one end, and connect the Luer lock at the other end.
- Prepare the AM, OM (removing BMP7), and FMCM mentioned in step 2.11. Additionally, prepare the common shared medium (SM; phenol red-free DMEM, 1x antibiotic-antimycotic, 1x Na-pyruvate, 1x ITS, 40 µg/mL proline, 50 µg/mL ascorbic acid, and 0.5 ng/mL transforming growth factor β3) to be used for the miniJoint culture. Load 35 mL of each medium into the medium reservoirs.
- Use straight forceps to transfer the osteochondral unit from the dual-flow bioreactor to the right well of the miniJoint bioreactor. Transfer the adipose tissue insert and fibrous tissue insert into the left and middle wells, respectively. Cap all the wells with sterilized lids.
- Connect the inlets of the miniJoint chip to the medium reservoirs and the outlets to syringes (Figure 3A-C).
- Mount the syringes onto a syringe pump (Figure 3C), and transfer the pump and chips to an incubator. The medium reservoirs are kept on ice outside the incubator.
- Operate the pump in the withdraw mode, drawing the medium from the medium reservoir into the miniJoint bioreactor chamber. This miniJoint culture process lasts 28 days.
- To model joint inflammation and cartilage degeneration, add interleukin 1β (IL-1β) to the fibrogenic medium stream at 10 ng/mL. Replace the syringes on the third day of IL-1β treatment, and provide fresh IL-1β to the fibrogenic medium. The treatment lasts for 7 days.
- During the drug testing step, after 3 days of IL-1β treatment, administer the drug either in the shared medium, simulating an "intraarticular administration" (Figure 3D) when the drug is used locally in the knee joint, or in all the medium types, simulating a "systemic administration" (Figure 3E) when the drug acts on the knee joint through the circulation.
- Collect individual tissues for analysis after 7 days of IL-1β treatment whether or not the samples underwent drug treatment for the previous 4 days.
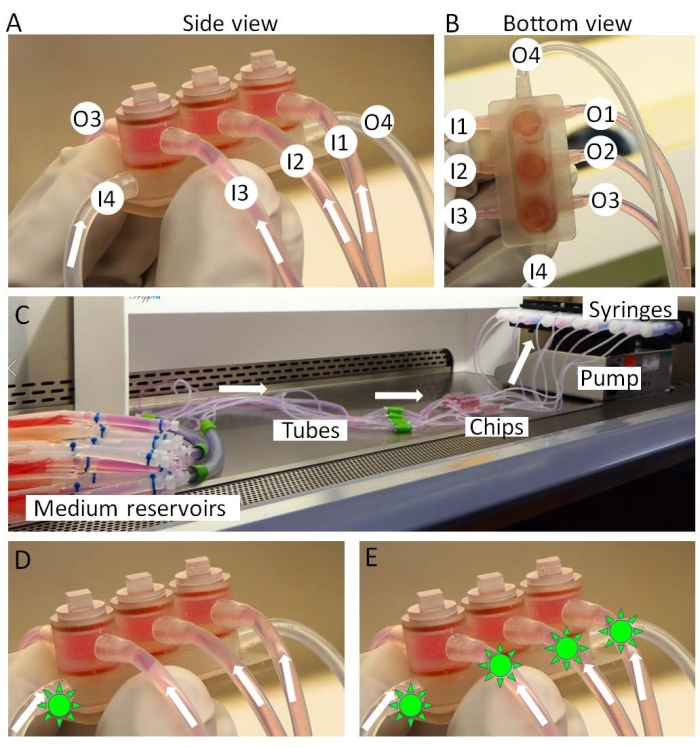
Figure 3: Assembly of the miniJoint. (A,B) Tissue-specific media are introduced from inlets 1-3 (I1-3) and moved out from outlets 1-3 (O1-3). The shared medium is perfused from I4 to O4. (C) The full setup of the miniJoint culture. Drugs (green sun like shapes) can be either introduced into (D) the shared medium only or (E) all the mediums to respectively simulate "intraarticular administration" or "systemic administration". Please click here to view a larger version of this figure.
4. Individual tissue collection
- Use sterile curved forceps to remove the inserts.
- Push a biopsy punch through the center of the insert to remove the gel, and place the gel in PBS.
- Cut the osteochondral gels in half when assessing the gene expression.
NOTE: Since the osteochondral gel consists of two tissue types, it is important to separate the osteogenic and chondrogenic cells. - Collect the conditioned media and tissues for various experiments.
- Collect around 1.5 mL from each medium source.
- Flash-freeze the conditioned media in liquid nitrogen after centrifuging at 14,000 x g for 10 min and discarding the sediment.
- For histological staining and immunostaining, first fix the OC and SFT samples in 10% buffered formalin, dehydrate them in ethanol of ascending concentrations, clear them in xylene, embed them in paraffin, and finally section them at a thickness of 6 µm.
- For the AT microtissues, fix the samples in 10% buffered formalin, and directly stain them with Oil Red O solution or BODIPY.
Results
All the tissues of the miniJoint were collected to analyze their phenotypes following 28 days of culture in the miniJoint (Figure 4A). This has been detailed in our previous publication7.
Through the use of RT-qPCR, immunostaining, and histological staining, it was confirmed that the tissue-specific phenotypes were well maintained for the individual microtissues (Figure 4). For example, the osseous component of...
Discussion
In this article, we present a protocol for creating a knee joint-on-a-chip system, in which bone, cartilage, adipose tissue, and synovium-like tissues are formed from MSCs and co-cultured within a customized bioreactor. This multi-component, human cell-derived system with plug-and-play features represents a new tool for studying the pathogenesis of joint diseases and developing drugs.
Given that different tissues favor specific culture media, it is critical to provide the respective medium fo...
Disclosures
The authors declare no competing interests.
Acknowledgements
This research was primarily supported by funding from the National Institutes of Health (UG3/UH3TR002136, UG3/UH3TR003090). In addition, we thank Dr. Paul Manner (University of Washington) for providing the human tissue samples and Dr. Jian Tan for their help in isolating the MSCs and creating the cell pool.
Materials
| Name | Company | Catalog Number | Comments |
| 3-isobutyl-1-methylxanthine | Sigma -Aldrich | I17018-1G | |
| 6 well non-tissue culture plate | Corning Falcon® Plates | 351146 | |
| 24 well non-tissue culture plate | Corning Falcon® Plates | 351147 | |
| 30 mL syringes | BD Syringe Luer Lock Cascade Health | 302832 | |
| Alcian blue stain | EK Industries | 1198 | 1% w/v, pH 1.0 |
| Advanced DMEM | Gibco | 12491-015 | |
| αMEM | Gibco | 12571-063 | |
| Antibiotic-antimycotic | Gibco | 15240-062 | |
| Biopsy punch | Integra Miltex | 12-460-407 | |
| BODIPY® fluorophore | Molecular Probes | ||
| Bone morphogenic protein 7 (BMP7) | Peprotech | ||
| Curved forceps | Fisher Brand | 16100110 | |
| DMEM | Gibco | 11995-065 | Dulbecco’s Modified Eagle Medium |
| Dexmethasome | Sigma -Aldrich | 02-05-2002 | |
| E-Shell 450 photopolymer in | EnvisionTec | RES-01-4022 | |
| Fetal Bovine Serum | Gemini-Bio Products | 900-208 | |
| GlutaMAX | Gibco | 3505-061 | |
| gelatin from bovine skin | Hyclone | 1003372809 | |
| Hank’s Balanced Salt Solution | Sigma -Aldrich | SH30588.02 | |
| indomethacin | Sigma -Aldrich | I7378-56 | |
| Insulin-Transferrin-Selenium-Ethanolamine (ITS) | Gibco | 51500-056 | |
| interleukin 1β | Peprotech | 200-01B | |
| Leur-loc connectors | Cole-Parmer Instruments | 45508-50 | |
| L-proline | Sigma -Aldrich | 115388-93-7 | |
| β-glycerophosphate | Sigma -Aldrich | 1003129352 | |
| Medium bags | KiYATEC | FC045 | |
| Methacrylic Anhydride | Sigma -Aldrich | 102378580 | |
| Phosphate buffered Saline | Corning | 21-040-CM | |
| Pointed forceps | Fisher Brand | 12000122 | |
| Silicon mold | McMaster-Carr | RC00114P | |
| Silicon o-rings | McMaster-Carr | ZMCCs1X5 | 1mm x 5mm |
| SolidWorks | Dassault Systèmes SE, Vélizy-Villacoublay, France | ||
| Surgical Blades | Integra Miltex | 4-122 | |
| Syringe pump | Lagato210P, KD Scientific | Z569631 | 10 syringe racks |
| T-182 tissue culture flasks | Fisher Brand | FB012939 | |
| Tissue Culture Dish 150 mm | Fisher Brand | FB012925 | |
| Transforming Growth Factor Beta (TGF-β3) | Peprotech | 100-36E | |
| Trypsin | Gibco | 25200-056 | |
| UV Flashlight | KBS | KB70109 | 395 nm |
| Vida Desktop 3D Printer | EnvisionTec | ||
| Vitamin D3 | Sigma -Aldrich | 32222-06-3 | 1,25-dihydroxyvitamin D3 |
References
- Safiri, S., et al. Global, regional and national burden of osteoarthritis 1990-2017: A systematic analysis of the Global Burden of Disease Study 2017. Annals of the Rheumatic Diseases. 79 (6), 819-828 (2020).
- Lawrence, R. C., et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis and Rheumatism. 58 (1), 26-35 (2008).
- Makarczyk, M. J., et al. Current models for development of disease-modifying osteoarthritis drugs. Tissue Engineering. Part C, Methods. 27 (2), 124-138 (2021).
- He, Y., et al. Pathogenesis of osteoarthritis: risk factors, regulatory pathways in chondrocytes, and experimental models. Biology. 9 (8), 194 (2020).
- Ronaldson-Bouchard, K., Vunjak-Novakovic, G. Organs-on-a-chip: A fast track for engineered human tissues in drug development. Cell Stem Cell. 22 (3), 310-324 (2018).
- Lin, H., Sohn, J., Shen, H., Langhans, M. T., Tuan, R. S. Bone marrow mesenchymal stem cells: aging and tissue engineering applications to enhance bone healing. Biomaterials. 203, 96-110 (2019).
- Li, Z., et al. Human mesenchymal stem cell-derived miniature joint system for disease modeling and drug testing. Advanced Science. 9 (21), 2105909 (2022).
- Lin, H., Cheng, A. W., Alexander, P. G., Beck, A. M., Tuan, R. S. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Engineering. Part A. 20 (17-18), 2402-2411 (2014).
- Fairbanks, B. D., Schwartz, M. P., Bowman, C. N., Anseth, K. S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials. 30 (35), 6702-6707 (2009).
- Lin, H., Lozito, T. P., Alexander, P. G., Gottardi, R., Tuan, R. S. Stem cell-based microphysiological osteochondral system to model tissue response to interleukin-1β. Molecular Pharmaceutics. 11 (7), 2203-2212 (2014).
- Yin, B., et al. Hybrid macro-porous titanium ornamented by degradable 3D gel/nHA micro-scaffolds for bone tissue regeneration. International Journal of Molecular Sciences. 17 (4), 575 (2016).
- Lin, Z., et al. Osteochondral tissue chip derived from iPSCs: Modeling OA pathologies and testing drugs. Frontiers in Bioengineering and Biotechnology. 7, 411 (2019).
- Atukorala, I., et al. Synovitis in knee osteoarthritis: A precursor of disease. Annals of the Rheumatic Diseases. 75 (2), 390-395 (2016).
- Occhetta, P., et al. Hyperphysiological compression of articular cartilage induces an osteoarthritic phenotype in a cartilage-on-a-chip model. Nature Biomedical Engineering. 3 (7), 545-557 (2019).
- He, C., et al. Modeling early changes associated with cartilage trauma using human-cell-laden hydrogel cartilage models. Stem Cell Research and Therapy. 13 (1), 400 (2022).
- Elsissy, J. G., et al. Bacterial septic arthritis of the adult native knee joint: A review. JBJS Reviews. 8 (1), 0059 (2020).
- Romero-Lopez, M., et al. Macrophage effects on mesenchymal stem cell osteogenesis in a three-dimensional in vitro bone model. Tissue Engineering. Part A. 26 (19-20), 1099-1111 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved