A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Isolation of Next-Generation Gene Therapy Vectors through Engineering, Barcoding, and Screening of Adeno-Associated Virus (AAV) Capsid Variants
In This Article
Summary
AAV peptide display library generation and subsequent validation through the barcoding of candidates with novel properties for the creation of next-generation AAVs.
Abstract
Gene delivery vectors derived from Adeno-associated virus (AAV) are one of the most promising tools for the treatment of genetic diseases, evidenced by encouraging clinical data and the approval of several AAV gene therapies. Two major reasons for the success of AAV vectors are (i) the prior isolation of various naturally occurring viral serotypes with distinct properties, and (ii) the subsequent establishment of powerful technologies for their molecular engineering and repurposing in high throughput. Further boosting the potential of these techniques are recently implemented strategies for barcoding selected AAV capsids on the DNA and RNA level, permitting their comprehensive and parallel in vivo stratification in all major organs and cell types in a single animal. Here, we present a basic pipeline encompassing this set of complementary avenues, using AAV peptide display to represent the diverse arsenal of available capsid engineering technologies. Accordingly, we first describe the pivotal steps for the generation of an AAV peptide display library for the in vivo selection of candidates with desired properties, followed by a demonstration of how to barcode the most interesting capsid variants for secondary in vivo screening. Next, we exemplify the methodology for the creation of libraries for next-generation sequencing (NGS), including barcode amplification and adaptor ligation, before concluding with an overview of the most critical steps during NGS data analysis. As the protocols reported here are versatile and adaptable, researchers can easily harness them to enrich the optimal AAV capsid variants in their favorite disease model and for gene therapy applications.
Introduction
Gene transfer therapy is the introduction of genetic material in cells to repair, replace, or alter the cellular genetic material to prevent, treat, cure, or ameliorate disease. Gene transfer, both in vivo and ex vivo, relies on different delivery systems, non-viral and viral. Viruses have evolved naturally to efficiently transduce their target cells and can be used as delivery vectors. Amongst the different types of viral vectors employed in gene therapy, adeno-associated viruses have been increasingly used, owing to their lack of pathogenicity, safety, low immunogenicity, and most importantly their ability to sustain long-term, non-integrating expression1,2,3. AAV gene therapy has yielded considerable achievements over the past decade; three therapies have been approved by the European Medicines Agency and the US Food and Drug Administration for use in humans3,4. Several clinical trials are also underway to treat a variety of diseases, such as hemophilia, muscular, cardiac, and neurological diseases, as reviewed elsewhere3. Despite decades of advancement, the field of gene therapy has experienced a series of setbacks in recent years4, most importantly deaths in clinical trials5 that have been put on hold due to dose-limiting toxicities, particularly for tissues that are massive, such as muscle, or difficult to reach, such as brain6.
The AAV vectors currently being used in clinical trials belong to the natural serotypes with a few exceptions1. AAV engineering offers the opportunity to develop vectors with superior organ- or cell-specificity and efficiency. In the past two decades, several approaches have been successfully applied, such as peptide display, loop-swap, capsid DNA shuffling, error-prone PCR, and targeted design, to generate individual AAV variants or libraries thereof with diverse properties7. These are then subjected to multiple rounds of directed evolution to select the variants within them with the desired properties, as reviewed elsewhere1,3. Of all the capsid evolution strategies, peptide display AAV libraries have been the most widely used, due to some unique properties: they are relatively easy to generate, and they can achieve high diversity and high-throughput sequencing, which allows trailing their evolution.
The first successful peptide insertion AAV libraries were described almost 20 years ago. In one of the first, Perabo et al.8 constructed a library of modified AAV2 capsids, in which a pool of randomly generated oligonucleotides was inserted in a plasmid at a position that corresponds to amino-acid 587 of the VP1 capsid protein, in the three-fold axis protruding from the capsid. Using adenovirus co-infection, the AAV library was evolved through multiple rounds of selection, and the final re-targeted variants were shown to be capable of transducing cell lines refractory to the parental AAV28. Shortly thereafter, Müller et al.9 introduced the two-step system for library production, a significant improvement to the protocol. Initially, the plasmid library, together with an adenoviral helper plasmid, are used to produce an AAV library that contains chimeric capsids. This AAV shuttle library is used to infect cells at low multiplicity of infection (MOI), with the aim to introduce one viral genome per cell. Co-infection with adenovirus ensures the production of AAVs with a matching genome and capsid9. About a decade later, Dalkara10 used in vivo directed evolution to create the 7m8 variant. This variant has a 10 amino-acid insertion (LALGETTRPA), three of which act as linkers, and efficiently targets the outer retina after intravitreal injection10. This engineered capsid is an exceptional success story, as it is one of the few engineered capsids to make it to the clinic thus far11.
The field experienced a second boost with the introduction of next-generation sequencing (NGS) techniques. Two publications from Adachi et al.12 in 2014 and from Marsic et al.13 in 2015, showcased the power of NGS to track the distribution of barcoded AAV capsid libraries with high accuracy. A few years later, the NGS of barcoded regions was adapted to the peptide insertion region to follow the capsid evolution. Körbelin et al.14 performed an NGS-guided screening to identify a pulmonary-targeted AAV2-based capsid. The NGS analysis helped calculate three rating scores: the enrichment score between selection rounds, the general specificity score to determine tissue specificity, and finally the combined score14. The Gradinaru lab15 published the Cre-recombination-based AAV targeted evolution (CREATE) system in the same year, which facilitates a cell-type-specific selection. In this system, the capsid library carries a Cre-invertible switch, as the polyA signal is flanked by two loxP sites. The AAV library is then injected in Cre mice, where the polyA signal is inverted only in Cre+ cells, providing the template for binding of a reverse PCR primer with the forward primer within the capsid gene. This highly specific PCR rescue enabled the identification of the AAV-PHP.B variant that can cross the blood-brain barrier15. This system was further evolved into M-CREATE (Multiplexed-CREATE), in which NGS and synthetic library generation were integrated in the pipeline16.
An improved RNA-based version of this system from the Maguire lab17, iTransduce, allows selection on the DNA level of capsids that functionally transduce cells and express their genomes. The viral genome of the peptide display library comprises a Cre gene under the control of a ubiquitous promoter and the capsid gene under the control of the p41 promoter. The library is injected in mice that have a loxP-STOP-loxP cassette upstream of tdTomato. Cells transduced with AAV variants that express the viral genome and therefore Cre express tdTomato and, in combination with cell markers, can be sorted and selected17. Similarly, Nonnenmacher et al.18 and Tabebordbar et al.19 placed the capsid gene library under the control of tissue-specific promoters. After injection in different animal models, viral RNA was used to isolate the capsid variants.
An alternative approach is to use barcoding to tag capsid libraries. The Björklund lab20 used this approach to barcode peptide insertion capsid libraries and developed the barcoded rational AAV vector evolution (BRAVE). In one plasmid, the Rep2Cap cassette is cloned next to an inverted terminal repeats (ITR)-flanked, yellow fluorescent protein (YFP)-expressing, barcode-tagged transgene. Using loxP sites between the end of cap and the beginning of the barcode, an in vitro Cre recombination generates a fragment small enough for NGS, thereby allowing the association of peptide insertion with the unique barcode (look-up table, LUT). AAV production is performed using the plasmid library and the barcodes expressed in the mRNA are screened after in vivo application, again with NGS20. When the capsid libraries comprise variants of the whole capsid gene (i.e., shuffled libraries), long-read sequencing needs to be used. Several groups have used barcodes to tag these diverse libraries, which enables NGS with higher read depth. The Kay lab21 tagged highly diverse capsid shuffled libraries with barcodes downstream of the cap polyA signal. In a first step, a barcoded plasmid library was generated, and the shuffled capsid gene library was cloned into it. Then a combination of MiSeq (short read, higher read depth) and PacBio (long read, lower read depth) NGS as well as Sanger sequencing was used to generate their LUT21. In 2019, Ogden and colleagues from the Church lab22 delineated the AAV2 capsid fitness for multiple functions using libraries that had single point mutations, insertions, and deletions in every position, which ultimately enabled machine-guided design. For the generation of the library, smaller fragments of the capsid gene were synthesized, tagged with a barcode, next-generation sequenced, and then cloned into the full capsid gene. The NGS data were used to generate a LUT. The library was then screened using just the barcodes and short read sequencing, which in turn allows higher read depth22.
Barcoded libraries have been predominantly used to screen a pool of known, natural, and engineered variants following several rounds of selection of capsid libraries or independent of a capsid evolution study. The advantage of such libraries is the opportunity to screen multiple capsids, whilst reducing animal numbers and minimizing variation between animals. The first studies that introduced this technology to the AAV field were published almost a decade ago. The Nakai lab12 tagged 191 double alanine mutants covering amino acids 356 to 736 on the VP1 from AAV9 with a pair of 12-nucleotide barcodes. Using NGS, the library was screened in vivo for galactose binding and other properties12. Marsic and colleagues delineated the biodistribution of AAV variants using also a double-barcorded analysis 1 year later13. A more recent study in non-human primates compared the biodistribution in the central nervous system of 29 capsids using different routes of delivery23. Our lab has recently published barcoded AAV library screens of 183 variants that included natural and engineered AAVs. These screens on the DNA and RNA level led to the identification of a highly myotropic AAV variant24 in mice as well as others displaying a high cell-type specificity in the mouse brain25.
Here, we describe the methodology used in this work and expand on it to include screening of AAV peptide display libraries. This comprises the generation of AAV2 peptide display libraries, a digital droplet PCR (dd-PCR) method for quantification, and finally an NGS pipeline to analyze the AAV variants, based in part on the work by Weinmann and colleagues24. Finally, a description of the generation of barcoded AAV libraries and the NGS pipeline used in the same publication is provided.
Protocol
1. AAV2 random 7-mer peptide display library preparation
NOTE: For the preparation of an AAV2 random peptide display library, synthesize the degenerate oligonucleotides as single-stranded DNA, convert it to double-stranded DNA, digest, ligate to the acceptor plasmid, and electroporate.
- Design of degenerate oligonucleotides
- Order the degenerate oligonucleotides and avoid codon bias. In the oligonucleotide 5' CAGTCGGCCAG AG W GGC (X01)7 GCCCAGGCGGCTGACGAG 3', X01 corresponds to 20 codons, each encoding one of the 20 amino acids. The W can be A or T, producing the codons AGA or AGT, which encode the amino acids arginine (R) or serine (S).
- Order the amplification primer: 5' CTCGTCAGCCGCCTGG 3' (see Figure 1 for details). This produces the following protein insert: R/S G X7. The theoretical diversity is calculated as follows: 1 x 2 x 207 = 2.56 x 109 unique variants.
NOTE: It should be noted that this diversity might be restricted by the transformation efficiency.
- Second-strand synthesis
- Resuspend both the oligonucleotides (degenerate oligonucleotides and amplification primer) to a 100 µM final concentration with TE buffer.
- For the PCR reaction, set up a 50 µL reaction with 1 µL of each primer, 10 µL of the buffer, 1.5 µL of DMSO, 0.5 µL of dNTPs (10 mM), 0.5 µL of Hi-fidelity Hot Start Polymerase II, and 35.5 µL of nuclease-free water.
- Transfer the reaction to a thermocycler and run a pre-incubation step for 10 s at 98 °C, followed by three cycles of 10 s at 98 °C, 30 s at 59 °C, and 10 s at 72 °C, then 5 min at 72 °C and a final cooling step.
- Purify the reaction using a nucleotide removal kit and elute in 100 µL of nuclease-free water.
- Confirm the efficiency of the second-strand synthesis by analysis on a Bioanalyzer (see Figure 2). Analyze the size and purity of the double-stranded insert by loading 1 µL of the reaction to a microfluidic chip from a DNA 1000 Reagents kit according to the manufacturer's instructions. This kit is optimized to measure the size and concentration of double-stranded DNA fragments from 25-1,000 bps.
- Resuspend both the oligonucleotides (degenerate oligonucleotides and amplification primer) to a 100 µM final concentration with TE buffer.
- Digestion of insert and plasmid vector
- Digest 85 µL of the purified insert with 10 µL of 10x buffer and 5 µL of BglI enzyme in a final 100 µL reaction volume (see Figure 1 for details). Incubate at 37 °C overnight. Purify using a nucleotide removal kit, elute in 50 µL of nuclease-free water, and quantify using the type "Oligo DNA" in a spectrophotometer.
- Digest 10 µg of a replication-competent AAV plasmid (pRep2Cap2_PIS)26 (ITR-flanked viral genome) with 20 µL of 10x buffer and 10 µL of SfiI enzyme in a final 200 µL reaction volume (see Figure 1 for details). Incubate at 50 °C overnight. Purify the vector on a 1% agarose gel using the gel extraction kit followed by an additional purification step using a DNA purifying kit. Quantify the concentration in a spectrophotometer.
- Ligation of insert to vector
- Ligate 955 ng of plasmid vector with 45 ng of insert with 2 µL of buffer and 2 µL of ligase in a 20 µL ligation reaction. Incubate at 16 °C overnight, followed by 10 min at 70 °C to heat-inactivate the ligase.
- Transformation, complexity calculation, and plasmid library preparation
- Purify the reaction with a DNA purifying kit following the manufacturer's instructions. Elute the reaction in about 80% of the starting volume of nuclease-free water and store on ice for subsequent transformation.
- Transform electrocompetent cells: thaw one vial of electrocompetent cells on ice for 10 min. Then add 1-2 µL of the purified ligation reaction to 30 µL (one vial) of electrocompetent cells and mix by gently tapping. Next, carefully pipette the cell/DNA mixture to a pre-chilled 1 mm gap electroporation cuvette without introducing air bubbles.
- Electroporate using the following settings: 1800 V, 600 Ω, and 10 µF. Within 10 s of the electroporation pulse, add 970 µL of pre-warmed recovery media (provided with the electrocompetent cells) to the cuvette and mix by pipetting. Lastly, transfer the cells to a micro centrifuge tube and incubate for 1 h at 37 °C at 250 rpm. To achieve a desired diversity, perform 10-100 reactions, and after incubation, pool all reactions in one tube.
- Calculate the diversity by diluting 10 µL of the pooled transformations 10-, 100-, or 1,000-fold in PBS and spread 100 µL on nutrient agar plates containing the appropriate antibiotic (75 mg/mL of ampicillin). Incubate the agar plates overnight at 37 °C and then count the colonies on the agar plates.
- Calculate the theoretical diversity as follows:
Theoretical maximal diversity = 10 x dilution factor x number of colonies x number of electroporation reactions.
NOTE: To confirm the library quality, sequence at least 20 colonies by Sanger sequencing. Most clones should contain an insert, and all should be unique. - Inoculate 400-1,000 mL of LB medium containing the appropriate antibiotic with the rest of the pooled transformations and incubate overnight at 37 °C, 180 rpm.
- Preparation of plasmid library
- From the overnight culture, prepare a glycerol stock (mix equal volumes of bacterial culture and 50% glycerol solution in nuclease-free water and freeze at -80 °C) and purify the plasmid library using a plasmid maxi kit.
- Production of AAV viral library
- Prepare the viral library as previously described27. Transfect the plasmid library (pRep2Cap2_PI, peptide insert) together with an adeno-helper plasmid to HEK293T cells using a transfection reagent such as polyethylenimine (PEI).
- Collect the cells after 3 days and subject them to three cycles of freeze-thaw. Purify the viral lysate using cesium chloride gradient ultracentrifugation, followed by buffer exchange to PBS, and finally concentrate the viral particles.
- AAV vector titration using dd-PCR
- Serially dilute 2 µL of the AAV vector stock in 198 µL of nuclease-free water to yield a 1:106 final dilution. Mix thoroughly each time using a 200 µL pipette. Add one no-template control (NTC) as a negative control.
NOTE: Additional lower or higher dilutions may be assayed (1:105-1:107). - Prepare a 20x primer-probe mix. Add 3.6 µL of each of the 100 µM primers (forward and reverse, Rep2, and ITR), 1 µL each of the 100 µM dd-PCR probes (Rep2 and ITR), and 3.6 µL of nuclease-free water to a 1.5 mL centrifuge tube.
NOTE: The AAV library is measured using a transgene-targeted primer-probe set (Rep2) detected with a FAM-labeled probe, and an ITR-targeted primer-probe set detected with a HEX-labeled probe. - Prepare a 22 µL PCR reaction by adding 5.5 µL of sample, 1.1 µL of 20x primer-probe mix, 11 µL of dd-PCR supermix for probes (no dUTP), and 4.4 µL of nuclease-free water. This yields concentrations of 900 nM and 250 nM for the primers and the probe, respectively.
- Generate the droplets using a droplet generator, transfer the reaction to a 96-well plate, place the plate into a thermocycler, and run a denaturation step for 10 min at 94 °C, followed by 40 cycles of 30 s at 94 °C and 1 min at 58 °C. Next, heat-inactivate the polymerase for 10 min at 98 °C and add a final cooling step. Read the reactions in a droplet reader and proceed to the analysis28.
- Open the saved dd-PCR plate file using the analysis software. Use the threshold tool in the 1D Amplitude tab (fluorescence amplitude vs. event number) to separate the negative and positive droplets for each channel, using the NTC as a guide, and export the data to a csv file.
- To calculate the vector concentration, first calculate the correction factor CF using the formula:
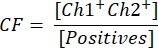
CF determines the proportion of droplets positive for the transgene [Positives] that are positive for both, transgene and ITR [Ch1+ Ch2+], to ensure the detection of functional vector particles. The final vector concentration c can now be calculated using the following equation:
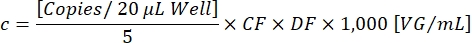
DF is the dilution factor (1:105-1:107 as determined earlier). The copies per 20 µL/well reaction correspond to 5 µL of the diluted sample. The factor 1,000 corrects the scale to VG/mL (viral genome/mL). An exemplary titration result is demonstrated in Table 1 and Figure 3.
- Serially dilute 2 µL of the AAV vector stock in 198 µL of nuclease-free water to yield a 1:106 final dilution. Mix thoroughly each time using a 200 µL pipette. Add one no-template control (NTC) as a negative control.
- Analysis of the AAV viral library by NGS
- Amplify the 96-nucleotide peptide insertion fragment by setting up a 20 µL PCR reaction using a proof-reading polymerase kit (2x; see Figure 4). Add 1 µL of AAV stock containing 1 x 108 vg, 0.5 µL of each of 100 µM primer (NGS_forward and NGS_reverse), and 10 µL of the enzyme mix to the reaction. Adjust the final volume to 20 µL with nuclease-free water.
- Transfer the reaction to a thermocycler and run a denaturation step for 3 min at 98 °C, followed by 30-35 cycles of 10 s at 98 °C, 10 s at 59 °C, and 20 s at 72 °C, followed by 5 min at 72 °C and a final cooling step.
- Purify the samples using a PCR purification kit. Quantify the concentration in a spectrophotometer and run a 3% agarose gel to verify purity and fragment size.
- Process the PCR fragments using the library system for low complexity samples kit according to the manufacturer's instructions for the preparation of an NGS library. Perform the end repair reaction with 30 ng of PCR fragment, followed by adaptor ligation and PCR amplification for 10 cycles. Use the PCR purification kit for the purification of the reactions.
- Process the final products on a Bioanalyzer to verify the size and purity, using a DNA reagents kit according to the manufacturer's instructions.
- Quantify the amplicons using a fluorometer and pool them. Quantify the final pooled NGS library again on a fluorometer (according to the manufacturer's instructions) and verify the quality on a Bioanalyzer.
- Sequence the NGS libraries in a single-end (SE) mode, using a 75-cycle high output kit, with a read length of 84 and an index 1 of 8.
NOTE: Sequencing of the examples in this article was performed at the GeneCore facility of EMBL Heidelberg (http://www.genecore.embl.de/). - Analyze the NGS sequencing data with Python 3 and biopython. The files can be found at https://github.com/grimmlabs/AAV_GrimmLab_JoVE2022 (alternatively at https://doi.org/10.5281/zenodo.7032215). The NGS analysis is composed of two steps.
- In the first step, search the sequence files for sequences that satisfy certain criteria (presence of recognition sequences flanking the insertion site) (see Figure 4, step 1.9.8.5.). This is done using a script (Script#1) and a configuration file that provides the information needed. Once the correct sequence is identified, the program extracts and stores the sequence in the output file, which is a txt file with the same name as the sequencing file.
- The second step is the analysis of the output files. The sequences in the library start with any of six nucleotides (AGWggc, W =A/T) in the nine amino-acid insert. Based on this start sequence, the peptide is translated. This generates the output files that contain the peptide variants (PVs).
- Prepare two folders: Script and Data. To the Data folder, copy the gzip-compressed files resulting from the sequencing. To the Script folder, copy the following files, Python file: Script#1_DetectionExtraction_JoVE_Py3.py; Python file: Script#2_PV_extraction_and_ranking_Py3.py; Configuration file: Barcode_Script_JoVE.conf; and Look-up table (LUT) file: Zuordnung.txt.
- Before running the scripts, edit the following files in the Script folder. Open the "Zuordnung.txt" file and add in two tab-separated columns, the names of the gzip files (column 1), and the desired final name (column 2; tab-separated values).
NOTE: Sample txt files are found in the GitHub folder "PV_analysis_script". The files provided in the GitHub folder are prepared for the analysis of three sample data from the above library: xaa.txt.gz, xab.txt.gz, and xac.txt.gz. The output files are also provided. - Change the following variables in the configuration file "Barcode_Script_JoVE.conf":
my_dir = "~/Data/"
filename_sample_file = "~/Script/Zuordnung.txt"
The sequence-specific variables: BCV_size = 27, BCVleft = TCCAGGGCCAG, BCVright = GCCCAGG, BCVloc = 30, BCVmargin = 8, BCVleft_revcomp = GCCGCCTGGGC, BCVright_revcomp = CTGGCCC, and BCVloc_revcomp = 41 (see Figure 4 for details). - Use the following command to call the variant sequence detection and extraction:
>python3 ~/Script#1_DetectionExtraction_JoVE_Py3.py ~/Barcode_Script_JoVE.conf
NOTE: The output are txt files with the extracted DNA sequences and their numbers of reads. The header of this file contains statistical data (i.e., the total number of reads and the extracted reads). These data are transferred to the next files. These txt data are the input files for Script#2, in which the DNA sequences are translated, ranked, and analyzed. - Perform PV extraction and analysis using the following command:
>python3 ~/Script#2_PV_extraction_and_ranking_Py3.py ~/Barcode_Script_JoVE.conf - Analyze the text output files of Script#2. The output files of Script#2 are named using the second column of the LUT in "Zuordnung.txt" with extensions based on the type of analysis.
NOTE: Ensure that the three output files contain statistical data in the first rows ("# of Valid PV reads", "# of Invalid PV reads", and "# of unique PV reads"), a first column with the index of each DNA sequence from the input txt files (output of Script#1), and the following columns: (1) "…analyzed_all.csv": "Sample:" (DNA sequence), "#" (number of reads), "Frw or Rev" (forward or reverse read), and "PVs" (translated peptide sequence). The invalid sequences have "NA" and "not valid" in the last two columns. (2) "…analyzed_validSeq.csv": same as the previous file, filtered for valid sequences. (3) "…analyzed_PV.csv": "PVs" (translated peptide sequence), "#" (number of reads), and "count" (the frw and rev counts in the previous files are merged and the count is given 1 or 2). - Visualize the output files using available software based on the user's needs.
2. AAV2 random 7-mer peptide display library selection
- Use the AAV library after quantification and quality-control (section 1) for directed evolution in a model of choice to iteratively select for candidates with desired properties (See Figure 5)16,18,21.
NOTE: These candidates are then used for the generation of a barcoded library as described below in section 3.
3. Barcoded AAV capsid library preparation and analysis
NOTE: Following the identification of a set of potentially specific and efficient AAV capsids in the peptide display screen, verify the functionality of the identified peptide sequences and compare them with a set of commonly used or well-described reference AAV capsid variants. To do this, the capsid sequence is inserted into a Rep/Cap helper construct without ITRs.
- Production of barcoded AAV library
- Perform recombinant AAV production for each capsid variant using the three-plasmid system, as previously described24.
NOTE: To distinguish the different capsid variants, the ITR-flanked reporter transgene plasmid harbors a unique barcode of 15 nucleotides in length. The barcode is located at the 3' UTR (untranslated region) between the enhanced yellow fluorescent protein (EYFP) and the polyA signal (see Figure 6A). EYFP expression is driven by a strong ubiquitous cytomegalovirus (CMV) promoter that provides sufficient levels of RNA transcripts. - Design barcodes of 15 nucleotides in length with homopolymers of less than three nucleotides, GC content of <65%29, and a Hamming distance greater than four nucleotides24.
- Produce each capsid separately in combination with a transgene plasmid carrying a unique barcode. This way, each capsid variant is tagged with a distinct barcode that enables its specific tracking (see Figure 6B).
- Perform recombinant AAV production for each capsid variant using the three-plasmid system, as previously described24.
- AAV vector titration using dd-PCR
- Perform the AAV titration as previously described in section 1.8, by replacing the Rep2 primer pair with the YFP primer pair.
- Quantify the individual AAV productions and pool equal amounts of each production to generate the final barcoded library.
- Quantify the final library again to check the final concentration and quality (see Figure 7).
- Barcoded AAV library in vivo application
- Apply the barcoded AAV library systemically to the model system of choice (e.g. systemically in mice24).
- Collect ON- and OFF-target tissues (i.e., liver, lung, heart, diaphragm, smooth muscle, duodenum, pancreas, colon, biceps, ovaries, stomach, inner ear, kidney, abdominal aorta, thoracic aorta, brain, brown and white fat, and spleen) or cell types based on the experiment. Freeze them at -80 °C, extract the DNA/RNA, and apply NGS quantitation analysis, as described in the next section.
- DNA/RNA extraction
- Extract the DNA and RNA from the tissues of interest using the DNA/RNA Mini Kit.
- Place a small piece of the tissue of interest (1 mm3, about 5 mg) in a 2 mL reaction tube.
- Add 350 µL of lysis buffer mixed with β-mercaptoethanol (1%) and 5 mm steel beads to the tissue (handle samples with β-mercaptoethanol under a fume hood).
- Homogenize the tissue in a tissueLyser for 45 s at 40 Hz.
- Add 10 µL of proteinase K (10 mg/mL) and incubate for 15 min at 55 °C while shaking at 400 rpm.
- Centrifuge at 20,000 x g for 3 min at room temperature, collect the supernatant, and proceed with the manufacturer's protocol of the DNA/RNA Kit.
- Split the washing step into two steps with 350 µL of wash buffer in each step. In between these washing steps, digest the remnant DNA on the column with RNase-free DNase I. Add 80 µL of the DNase I solution, prepared according to the manufacturer's instruction, onto the column and incubate at room temperature for 15 min.
- Elute RNA/DNA from the column with nuclease-free water. Store the isolated RNA at -80 °C and the gDNA at -20 °C.
- cDNA synthesis
- Subject the RNA samples to another round of DNase I treatment of 15-30 min (for complete removal of contaminating DNA from the RNA samples) before the reverse-transcription reaction. Add 1 µL of the DNase I solution, 4 µL of buffer (provided with the kit), and nuclease-free water to a final volume of 40 µL to 212 ng of RNA. Incubate for 30 min at room temperature and heat inactivate at 70 °C for 10 min.
- Synthesize cDNA, using 150 ng of RNA using a kit according to the manufacturer's instructions. Include controls without the reverse transcriptase, to ensure the absence of contaminating viral DNA from the sample. The cDNA is stored at -20 °C.
NOTE: The amount of input RNA for optimal reverse transcription can vary depending on the tissue type and the expected transduction efficiency in the respective tissue.
- Analysis of AAV viral library (in-vivo) by NGS
- To achieve high sequencing depth at low cost, perform NGS via Illumina sequencing as previously described (section 1.9). Amplify the barcode sequence, and then ligate the sequencing adaptors to the amplicon.
- Due to the short-read length and the ligation of the sequencing adapters on both sides of the amplicon, when designing, check that the amplicon is sufficiently small to ensure presence of the barcode sequence within the NGS read. For the sequencing of the barcodes within the viral genomes and the viral transcripts, the PCR amplicon is designed to be 113 bp long (see Figure 8).
- Amplify the barcoded region with the primers BC-seq forward and BC-seq reverse. Prepare the following PCR reaction: 0.5 µL of Hi-fidelity DNA polymerase, 10 µL of 5x buffer, 0.25 µL of each 100 µM primer (BC-seq fw/BC-seq rv), and 1 µl of 10 mM dNTPs. Use 25 ng of the cDNA or DNA/reaction as a template and adjust the final volume to 50 µL with nuclease-free water.
- Prepare the PCR master-mix under a clean PCR hood to avoid contamination. Use the following cycling conditions: 30 s at 98 °C, followed by 40 cycles at 98 °C for 10 s and 72 °C for 20 s, and a final 5 min step at 72 °C.
- Include PCR controls to confirm the absence of contaminating DNA in the PCR master-mix. For the cDNA samples, include the controls without reverse transcriptase. Finally, include a sample with the AAV input library. This information will be used to generate the Normalization_Variant.txt file used in the analysis.
- Verify the size of the PCR fragment of each sample by gel electrophoresis before PCR purification. The latter is achieved by using either commercially available magnetic beads or column-based DNA purification systems (see Table of Materials).
- Prepare the NGS library using the library system for low complexity samples according to the manufacturer's instructions, as previously described in section 1.9.
- Determine the DNA concentration via the dsDNA HS Kit and analyze the quality of the library as previously described (section 1.9.6), followed by pooling. Quantify the pooled library on a fluorometer and assess the quality on a Bioanalyzer.
- Perform NGS sequencing as discussed in section 1.9.7.
- Quantify by qPCR the copy number of the transgene (viral genomes) and the housekeeping gene to assess the distribution of the pooled library between tissues or organs on the DNA.
- Set up a 30 µL qPCR reaction as follows, to determine the copy number of EYFP (transgene) and GAPDH (glyceraldehyde 3-phosphate dehydrogenase, housekeeping gene):
- Prepare a 60x primer/probe mix for EYFP (1.5 µM YFP_fw, 1.5 µM YFP_rv, and 0.6 µM YFP_probe; see Table of Materials). Use GAPDH primer/probe mix (see Table of Materials) to determine the copy number of the housekeeper gene. Set up the reaction on ice.
- Prepare a PCR master mix (15 µL, see Table of Materials) and add 60x primer/probe mix (0.5 µL) for all samples and standards (to calculate copy numbers for the standards, use the following link: http://cels.uri.edu/gsc/cndna.html). Set up the reaction on ice.
- Transfer 15.5 µL of the master mix into a 96-well plate and add 14.5 µL of sample (75 ng of total DNA concentration) or standard to the respective well. Seal the 96-well plate with foil, vortex, and spin briefly.
- Transfer 10 µL of each sample into a 384-well plate in duplicates. Seal the plate with foil and spin at 800 x g for 5 min at 4 °C.
- Incubate the reaction mix in a thermocycler using an initial temperature of 50 °C for 2 min, followed by an initial activation step of 10 min at 95 °C. Perform 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min24.
- To obtain the number of diploid genomes (dg), use the GAPDH copy number and divide by two. Then, take the value of the EYFP copy number and divide by the number of dg, resulting in vector genomes per diploid genome (vg/dg). Use this value to generate the Normalization_Organ.txt file for the bioinformatic analysis.
- Perform the analysis of the NGS sequencing data like Weinmann et al.24, using custom code in Python3 (https://github.com/grimmlabs/AAV_GrimmLab_JoVE2022). The workflow comprises the detection of barcode sequences guided by flanking sequences, their length and location (Script#1_BarcodeDetection.py), as well as analysis of barcode enrichment and distribution over the set of tissues (Script#2_BarcodeAnalysis.py).
- Detect barcode and assign them to AAV variants. Place the sequencing data as archived fastq files in one directory (e.g., "Data_to_analyze"). The sequencing data file for the input library is included in this directory and used only to calculate the capsid proportions in the input library.
- Before executing the script, create two tab-delimited text files: the capsid variants file (see example file "Variants.txt") with the barcode sequences assigned to AAV capsid variant names, and the contamination file (see "Contaminations.txt") with barcode sequences which come from possible contamination (other barcodes available in the lab, contributing to contamination).
- Finally, edit the configuration file "Barcode_Script.conf" to include the following information: path to folder with sequencing data (e.g., "Data_to_analyze"), sequence of flanking regions of the barcodes, their position, and window size for barcode detection (similar to 1.9.8.5, see Figure 8).
- Use the following command to call for barcode detection with provided paths to Script#1_BarcodeDetection.py and configuration files:
>python3 ~/Script#1_BarcodeDetection.py ~/Barcode_Script.conf
NOTE: The output of Script#1_BarcodeDetection.py execution is text files with read counts per capsid variant as well as the total number of reads recovered from the raw data. - Evaluate the distribution of barcoded AAV capsids among tissues or organs, by executing Script#2_BarcodeAnalysis.py together with the following txt files:
- In the "Zuordnung.txt" file, assign the name to each txt file obtained from the barcode detection run to a tissue/organ name: names of txt files in the first column and corresponding tissue/organ names in tab-delimited assignment.
NOTE: For an example, check in the "Example" folder (https://github.com/grimmlabs/AAV_GrimmLab_JoVE2022). Of note, the tissue/organ name can include characters defining cDNA or gDNA measurement and biological replicate number (M1, M2, etc.). - Create an "organs.txt" text file with the list of names for ON- and OFF-target organs, which correspond to the names given in the assignment "Zuordnung.txt" file (see "Example" folder: https://github.com/grimmlabs/AAV_GrimmLab_JoVE2022).
- Create "Normalization_Organ.txt" and "Normalization_Variant.txt" tab-delimited text files with normalized values for all capsid variants and all organs/tissues. In the first column of the "Normalization_Organ.txt" file, write the names given for each organ (as in the assignment file "Zuordnung.txt") and in the second column the normalization values for the corresponding tissues, generated in section 3.6.11.
- Fill the first column of the "Normalization_Variant.txt" file with the list of capsid names and the second column with the normalized values of the read counts for each capsid in the pooled library (normalization can be calculated based on the txt output file for the input library resulting from the first script).
- Edit the configuration file by specifying the full paths to all additional files mentioned above. Execute Script#2_BarcodeAnalysis.py as:
>python3 /Script#2_BarcodeAnalysis.py ~/Barcode_Script.conf
NOTE: The barcode analysis script outputs several files: text files with relative concentration (RC) values of capsid distribution within different tissues based on multiple normalization steps described earlier, and the spreadsheet file which combines text file data into merged matrix data. The latter can be used for cluster analysis and visualization. - Visualize the data and perform cluster analysis of the matrix data in order to distinguish capsid properties and evaluate their similarities based on RC profiles across tissues. Use the additional script PCA_heatmap_plot.R placed in the repository:
>Rscript --vanilla ~/PCA.R ~/relativeconcentration.xls
NOTE: The script takes relativeconcentration.xls files as input and generates two plots of hierarchical cluster heatmap and principal component analysis (PCA). - To modify plots (axes of heatmap, principal components of PCA) or png parameters (color, size, labeling), open the R script and follow the instructions provided in the commented sections.
- In the "Zuordnung.txt" file, assign the name to each txt file obtained from the barcode detection run to a tissue/organ name: names of txt files in the first column and corresponding tissue/organ names in tab-delimited assignment.
Results
Generation of an AAV2 peptide display library. As a first step toward the selection of engineered AAVs, the generation of a plasmid library is described. The peptide insert is produced by using degenerate primers. Reducing the combination of codons in those from 64 to 20 has the advantages of eliminating stop codons and facilitating NGS analysis, by reducing library diversity on the DNA but not the protein level. The oligonucleotide insert is purchased as single-stranded DNA (Figure ...
Discussion
In this protocol, the steps needed for peptide display AAV capsid engineering and for barcoded AAV library screening, as well as for bioinformatic analysis of library composition and capsid performance, are outlined. This protocol focuses on the steps that facilitate the bioinformatic analysis of these types of libraries, because most virology laboratories lag in programming skills to match their proficiency in molecular biology techniques. Both types of libraries have been extensively described in the literature, as out...
Disclosures
D.G. is a co-founder of AaviGen GmbH. D.G. and K.R. are inventors on a pending patent application related to the generation of immune-evading AAV capsid variants. The rest of the authors have nothing to disclose.
Acknowledgements
D.G. greatly appreciates support by the German Research Foundation (DFG) through the DFG Collaborative Research Centers SFB1129 (Projektnummer 240245660) and TRR179 (Projektnummer 272983813), as well as by the German Center for Infection Research (DZIF, BMBF; TTU-HIV 04.819).
Materials
| Name | Company | Catalog Number | Comments |
| Amplification primer | ELLA Biotech (Munich, Germany) | - | Second-strand synthesis of oligonucleotide insert |
| Agilent DNA 1000 Reagents | Agilent Technologies (Santa Clara, CA, USA) | 5067-1504 | DNA fragment validation |
| Agilent 2100 Bioanalyzer System | Agilent Technologies (Santa Clara, CA, USA) | G2938C | DNA fragment validation |
| AllPrep DNA/RNA Mini Kit | Qiagen (Venlo, Netherlands) | 80204 | DNA/RNA extraction |
| Agilent DNA 1000 Reagents | Agilent Technologies (Santa Clara, CA, USA) | 5067-1504 | NGS Library preparation |
| Agilent 2100 Bioanalyzer System | Agilent Technologies (Santa Clara, CA, USA) | G2938C | NGS Library preparation |
| BC-seq fw: | IDT (San Joce, CA, CA, USA) | ATCACTCTCGGCATGGACGAGC | NGS Library preparation |
| BC-seq rv: | IDT (San Joce, CA, CA, USA) | GGCTGGCAACTAGAAGGCACA | NGS Library preparation |
| β-Mercaptoethanol | Millipore Sigma (Burlington, MA, USA) | 44-420-3250ML | DNA/RNA extraction |
| BglI | New England Biolabs (Ipswich, MA, USA) | R0143 | Digestion of double-stranded insert |
| C1000 Touch Thermal Cycler | Bio-Rad (Hercules, CA, USA) | 1851196 | dd-PCR cycler |
| dNTPS | New England Biolabs (Ipswich, MA, USA) | N0447S | NGS Library preparation |
| ddPCR Supermix for probes (no dUTP) | Bio-Rad (Hercules, CA, USA) | 1863024 | dd-PCR supermix |
| Droplet Generation Oil for Probes | Bio-Rad (Hercules, CA, USA) | 1863005 | dd-PCR droplet generation oil |
| DG8 Cartridges for QX100 / QX200 Droplet Generator | Bio-Rad (Hercules, CA, USA) | 1864008 | dd-PCR droplet generation cartridge |
| DG8 Cartridge Holder | Bio-Rad (Hercules, CA, USA) | 1863051 | dd-PCR cartridge holder |
| Droplet Generator DG8 Gasket | Bio-Rad (Hercules, CA, USA) | 1863009 | dd-PCR cover for cartridge |
| ddPCR Plates 96-Well, Semi-Skirted | Bio-Rad (Hercules, CA, USA) | 12001925 | dd-PCR 96-well plate |
| E.cloni 10G SUPREME Electrocompetent Cells | Lucigen (Middleton, WI, USA) | 60081-1 | Electrocompetent cells |
| Electroporation cuvettes, 1mm | Biozym Scientific (Oldendorf, Germany) | 748050 | Electroporation |
| GAPDH primer/probe mix | Thermo Fischer Scientific (Waltham, MA, USA) | Mm00186825_cn | Taqman qPCR primer |
| Genepulser Xcell | Bio-Rad (Hercules, CA, USA) | 1652660 | Electroporation |
| High-Capacity cDNA Reverse Transcription Kit | Applied Biosystems (Waltham, MA, USA) | 4368814 | cDNA reverse transcription |
| ITR_fw | IDT (San Joce, CA, USA) | GGAACCCCTAGTGATGGAGTT (https://signagen.com/blog/2019/10/25/qpcr-primer-and-probe-sequences-for-raav-titration/) | dd-PCR primer |
| ITR_rv | IDT (San Joce, CA, USA) | CGGCCTCAGTGAGCGA (https://signagen.com/blog/2019/10/25/qpcr-primer-and-probe-sequences-for-raav-titration/) | dd-PCR primer |
| ITR_probe | IDT (San Joce, CA, USA) | HEX-CACTCCCTCTCTGCGCGCTCG-BHQ1 (https://signagen.com/blog/2019/10/25/qpcr-primer-and-probe-sequences-for-raav-titration/) | dd-PCR probe |
| Illumina NextSeq 500 system | Illumina Inc (San Diego, CA, USA) | SY-415-1001 | NGS Library sequencing |
| KAPA HiFi HotStart ReadyMix (2X)* | Roche AG (Basel, Switzerland) | KK2600 07958919001 | NGS sample prepration |
| MagnaBot 96 Magnetic Separation Device | Promega GmbH (Madison, WI, USA) | V8151 | Sample prepration for NGS library |
| NanoDrop 2000 spectrophotometer | Thermo Fischer Scientific (Waltham, MA, USA) | ND-2000 | Digestion of double-stranded insert |
| NGS_frw | Sigma-Aldrich (Burlinght, MA, USA) | GTT CTG TAT CTA CCA ACC TC | NGS primer |
| NGS_rev | Sigma-Aldrich (Burlinght, MA, USA) | CGC CTT GTG TGT TGA CAT C | NGS primer |
| NextSeq 500/550 High Output Kit (75 cycles) | Illumina Inc (San Diego, CA, USA) | FC-404-2005 | NGS Library sequencing |
| Ovation Library System for Low Complexity Samples Kit | NuGEN Technologies, Inc. (San Carlos, CA, USA) | 9092-256 | NGS Library preparation |
| PX1 Plate Sealer | Bio-Rad (Hercules, CA, USA) | 1814000 | dd-PCR plate sealer |
| Pierceable Foil Heat Seal | Bio-Rad (Hercules, CA, USA) | 1814040 | dd-PCR sealing foil |
| Phusion High-Fidelity DNA-Polymerase | Thermo Fischer Scientific (Waltham, MA, USA) | F530S | Second-strand synthesis of oligonucleotide insert |
| PEI MAX - Transfection Grade Linear Polyethylenimine Hydrochloride (MW 40,000) | Polysciences, Inc. (Warrington, PA, USA) | 24765-1G | AAV library preparation |
| ProNex Size-Selective Purification System | Promega GmbH (Madison, WI, USA) | NG2002 | Sample prepration for NGS library |
| Phusion Hot Start II Polymerase | Thermo Fischer Scientific (Waltham, MA, USA) | F549L | NGS Library preparation |
| Proteinase K | Roche AG (Basel, Switzerland) | 5963117103 | DNA/RNA extraction |
| pRep2Cap2_PIS | ITR-Rep2Cap2-ITR vector. Peptide insertion site within the Cap2 ORF, manufactured/prepared in the lab | ||
| QX200 Droplet Generator | Bio-Rad (Hercules, CA, USA) | 1864002 | dd-PCR droplet generator |
| QX200 Droplet Reader | Bio-Rad (Hercules, CA, USA) | 1864003 | dd-PCR droplet analysis |
| QIAquick Nucleotide Removal Kit | Qiagen (Venlo, Netherlands) | 28306 | Second-strand synthesis of oligonucleotide insert purification |
| QIAquick Gel Extraction Kit | Qiagen (Venlo, Netherlands) | 28704 | Plasmid vector purification |
| QIAGEN Plasmid Maxi Kit | Qiagen (Venlo, Netherlands) | 12162 | Plasmid library DNA preparation |
| Qiaquick PCR Purification kit | Qiagen (Venlo, Netherlands) | 28104 | Sample prepration for NGS library |
| Qubit fluorometer | Invitrogen (Waltham, MA, USA) | Q32857 | NGS Library preparation |
| Qubit dsDNA HS | Thermo Fischer Scientific (Waltham, MA, USA) | Q32851 | NGS Library preparation |
| QuantiFast PCR Master Mix | Qiagen (Venlo, Netherlands) | 1044234 | Taqman qPCR |
| rep_fw | IDT (San Joce, CA, USA) | AAGTCCTCGGCCCAGATAGAC | dd-PCR primer |
| rep_rv | IDT (San Joce, CA, USA) | CAATCACGGCGCACATGT | dd-PCR primer |
| rep_probe | IDT (San Joce, CA, USA) | FAM-TGATCGTCACCTCCAACA-BHQ1 | dd-PCR probe |
| RNase-free DNase | Qiagen (Venlo, Netherlands) | 79254 | DNA/RNA extraction |
| SfiI | New England Biolabs (Ipswich, MA, USA) | R0123 | Digestion of vector |
| 5 mm, steel Beads | Qiagen (Venlo, Netherlands) | 69989 | DNA/RNA extraction |
| TRIMER-oligonucleotides | ELLA Biotech (Munich, Germany) | - | Degenerate oligonucleotide |
| T4 Ligase | New England Biolabs (Ipswich, MA, USA) | M0202L | Plasmid library ligation |
| TissueLyserLT | Qiagen (Venlo, Netherlands) | 85600 | DNA/RNA extraction |
| YFP_fw | IDT (San Joce, CA, USA) | GAGCGCACCATCTTCTTCAAG | dd-PCR primer |
| YFP_rv | IDT (San Joce, CA, USA) | TGTCGCCCTCGAACTTCAC | dd-PCR primer |
| YFP_probe | IDT (San Joce, CA, USA) | FAM-ACGACGGCAACTACA-BHQ1 | dd-PCR probe |
| Zymo DNA Clean & Concentrator-5 (Capped) | Zymo research (Irvine, CA, USA) | D4013 | Vector and Ligation purification |
References
- Wang, D., Tai, P. W. L., Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nature Reviews Drug Discovery. 18 (5), 358-378 (2019).
- Muhuri, M., Levy, D. I., Schulz, M., McCarty, D., Gao, G. Durability of transgene expression after rAAV gene therapy. Molecular Therapy. 30 (4), 1364-1380 (2022).
- Li, C., Samulski, R. J. Engineering adeno-associated virus vectors for gene therapy. Nature Reviews Genetics. 21 (4), 255-272 (2020).
- Kuzmin, D. A., et al. The clinical landscape for AAV gene therapies. Nature Reviews Drug Discovery. 20 (3), 173-174 (2021).
- Mullard, A. Gene therapy community grapples with toxicity issues, as pipeline matures. Nature Reviews Drug Discovery. 20 (11), 804-805 (2021).
- Nature Biotechnology. Gene therapy at the crossroads. Nature Biotechnology. 40 (5), 621 (2022).
- Becker, P., et al. Fantastic AAV Gene Therapy Vectors and How to Find Them-Random Diversification, Rational Design and Machine Learning. Pathogens. 11 (7), 756 (2022).
- Perabo, L., et al. In vitro selection of viral vectors with modified tropism: the adeno-associated virus display. Molecular Therapy. 8 (1), 151-157 (2003).
- Muller, O. J., et al. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nature Biotechnology. 21 (9), 1040-1046 (2003).
- Dalkara, D., et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Science Translational Medicine. 5 (189), (2013).
- Sahel, J. A., et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nature Medicine. 27 (7), 1223-1229 (2021).
- Adachi, K., Enoki, T., Kawano, Y., Veraz, M., Nakai, H. Drawing a high-resolution functional map of adeno-associated virus capsid by massively parallel sequencing. Nature Communications. 5, 3075 (2014).
- Marsic, D., Mendez-Gomez, H. R., Zolotukhin, S. High-accuracy biodistribution analysis of adeno-associated virus variants by double barcode sequencing. Molecular Therapy-Methods & Clinical Development. 2, 15041 (2015).
- Korbelin, J., et al. Pulmonary targeting of adeno-associated viral vectors by next-generation sequencing-guided screening of random capsid displayed peptide libraries. Molecular Therapy. 24 (6), 1050-1061 (2016).
- Deverman, B. E., et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nature Biotechnology. 34 (2), 204-209 (2016).
- Ravindra Kumar, S., et al. Multiplexed Cre-dependent selection yields systemic AAVs for targeting distinct brain cell types. Nature Methods. 17 (5), 541-550 (2020).
- Hanlon, K. S., et al. Selection of an efficient AAV vector for robust CNS transgene expression. Molecular Therapy-Methods & Clinical Development. 15, 320-332 (2019).
- Nonnenmacher, M., et al. Rapid evolution of blood-brain-barrier-penetrating AAV capsids by RNA-driven biopanning. Molecular Therapy-Methods & Clinical Development. 20, 366-378 (2021).
- Tabebordbar, M., et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell. 184 (19), 4919-4938 (2021).
- Davidsson, M., et al. A systematic capsid evolution approach performed in vivo for the design of AAV vectors with tailored properties and tropism. Proceedings of the National Academy of Sciences. 116 (52), 27053-27062 (2019).
- Pekrun, K., et al. Using a barcoded AAV capsid library to select for clinically relevant gene therapy vectors. Journal of Clinical Investigation Insight. 4 (22), (2019).
- Ogden, P. J., Kelsic, E. D., Sinai, S., Church, G. M. Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science. 366 (6469), 1139-1143 (2019).
- Kondratov, O., et al. A comprehensive study of a 29-capsid AAV library in a non-human primate central nervous system. Molecular Therapy. 29 (9), 2806-2820 (2021).
- Weinmann, J., et al. Identification of a myotropic AAV by massively parallel in vivo evaluation of barcoded capsid variants. Nature Communications. 11 (1), 5432 (2020).
- Kremer, L. P. M., et al. High throughput screening of novel AAV capsids identifies variants for transduction of adult NSCs within the subventricular zone. Molecular Therapy-Methods & Clinical Development. 23, 33-50 (2021).
- Borner, K., et al. Pre-arrayed pan-AAV peptide display libraries for rapid single-round screening. Molecular Therapy. 28 (4), 1016-1032 (2020).
- Kienle, E., et al. Engineering and evolution of synthetic adeno-associated virus (AAV) gene therapy vectors via DNA family shuffling. Journal of Visualized Experiments. (62), e3819 (2012).
- Furuta-Hanawa, B., Yamaguchi, T., Uchida, E. Two-dimensional droplet digital PCR as a tool for titration and integrity evaluation of recombinant adeno-associated viral vectors. Human Gene Therapy Methods. 30 (4), 127-136 (2019).
- Lyons, E., Sheridan, P., Tremmel, G., Miyano, S., Sugano, S. Large-scale DNA barcode library generation for biomolecule identification in high-throughput screens. Scientific Reports. 7 (1), 13899 (2017).
- Korbelin, J., et al. Optimization of design and production strategies for novel adeno-associated viral display peptide libraries. Gene Therapy. 24 (8), 470-481 (2017).
- Korbelin, J., Trepel, M. How to successfully screen random adeno-associated virus display peptide libraries in vivo. Human Gene Therapy Methods. 28 (3), 109-123 (2017).
- Herrmann, A. K., et al. A robust and all-inclusive pipeline for shuffling of adeno-associated viruses. American Chemical Society Synthetic Biology. 8 (1), 194-206 (2019).
- Choudhury, S. R., et al. In vivo selection yields AAV-B1 Capsid for central nervous system and muscle gene therapy. Molecular Therapy. 24 (7), 1247-1257 (2016).
- Buschmann, T., Bystrykh, L. V. Levenshtein error-correcting barcodes for multiplexed DNA sequencing. BMC Bioinformatics. 14, 272 (2013).
- Buschmann, T. DNABarcodes: an R package for the systematic construction of DNA sample tags. Bioinformatics. 33 (6), 920-922 (2017).
- Li, B., et al. A comprehensive mouse transcriptomic BodyMap across 17 tissues by RNA-seq. Scientific Reports. 7 (1), 4200 (2017).
- Clarner, P., et al. Development of a one-step RT-ddPCR method to determine the expression and potency of AAV vectors. Molecular Therapy-Methods & Clinical Development. 23, 68-77 (2021).
- Zolotukhin, S., Vandenberghe, L. H. AAV capsid design: A Goldilocks challenge. Trends in Molecular Medicine. 28 (3), 183-193 (2022).
- Brown, D., et al. deep parallel characterization of AAV tropism and AAV-mediated transcriptional changes via single-cell RNA sequencing. Frontiers in Immunology. 12, 730825 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved