A subscription to JoVE is required to view this content. Sign in or start your free trial.
Automation of the Micronucleus Assay Using Imaging Flow Cytometry and Artificial Intelligence
In This Article
Summary
The micronucleus (MN) assay is a well-established test for quantifying DNA damage. However, scoring the assay using conventional techniques such as manual microscopy or feature-based image analysis is laborious and challenging. This paper describes the methodology to develop an artificial intelligence model to score the MN assay using imaging flow cytometry data.
Abstract
The micronucleus (MN) assay is used worldwide by regulatory bodies to evaluate chemicals for genetic toxicity. The assay can be performed in two ways: by scoring MN in once-divided, cytokinesis-blocked binucleated cells or fully divided mononucleated cells. Historically, light microscopy has been the gold standard method to score the assay, but it is laborious and subjective. Flow cytometry has been used in recent years to score the assay, but is limited by the inability to visually confirm key aspects of cellular imagery. Imaging flow cytometry (IFC) combines high-throughput image capture and automated image analysis, and has been successfully applied to rapidly acquire imagery of and score all key events in the MN assay. Recently, it has been demonstrated that artificial intelligence (AI) methods based on convolutional neural networks can be used to score MN assay data acquired by IFC. This paper describes all steps to use AI software to create a deep learning model to score all key events and to apply this model to automatically score additional data. Results from the AI deep learning model compare well to manual microscopy, therefore enabling fully automated scoring of the MN assay by combining IFC and AI.
Introduction
The micronucleus (MN) assay is fundamental in genetic toxicology to evaluate DNA damage in the development of cosmetics, pharmaceuticals, and chemicals for human use1,2,3,4. Micronuclei are formed from whole chromosomes or chromosome fragments that do not incorporate into the nucleus following division and condense into small, circular bodies separate from the nucleus. Thus, MN can be used as an endpoint to quantify DNA damage in genotoxicity testing1.
The preferred method for quantifying MN is within once-divided binucleated cells (BNCs) by blocking division using Cytochalasin-B (Cyt-B). In this version of the assay, cytotoxicity is also assessed by scoring mononucleated (MONO) and polynucleated (POLY) cells. The assay can also be performed by scoring MN in unblocked MONO cells, which is faster and easier to score, with cytotoxicity being assessed using pre- and post-exposure cell counts to assess proliferation5,6.
Physical scoring of the assay has historically been performed through manual microscopy, since this permits visual confirmation of all key events. However, manual microscopy is challenging and subjective1. Thus, automated techniques have been developed, including microscope slide scanning and flow cytometry, each with their own advantages and limitations. While slide-scanning methods allow key events to be visualized, slides must be created at optimal cell density, which can be difficult to achieve. Additionally, this technique often lacks cytoplasmic visualization, which can compromise the scoring of MONO and POLY cells7,8. While flow cytometry offers high-throughput data capture, the cells must be lysed, thus not permitting the use of the Cyt-B form of the assay. Additionally, as a non-imaging technique, conventional flow cytometry does not provide visual validation of key events9,10.
Therefore, imaging flow cytometry (IFC) has been investigated to perform the MN assay. The ImageStreamX Mk II combines the speed and statistical robustness of conventional flow cytometry with the high-resolution imaging capabilities of microscopy in a single system11. It has been shown that by using IFC, high-resolution imagery of all key events can be captured and automatically scored using feature-based12,13 or artificial intelligence (AI) techniques14,15. By using IFC to perform the MN assay, the automatic scoring of many more cells compared to microscopy in a shorter amount of time is achievable.
This work deviates from a previously described image analysis workflow16 and discusses all steps required to develop and train a Random Forest (RF) and/or convolutional neural network (CNN) model using the Amnis AI software (henceforth referred to as "AI software"). All necessary steps are described, including populating ground truth data using AI-assisted tagging tools, interpretation of model training results, and application of the model to classify additional data, permitting calculation of genotoxicity and cytotoxicity15.
Protocol
1. Data acquisition using imaging flow cytometry
NOTE: Refer to Rodrigues et al.16 with the following modifications, noting that the acquisition regions using IFC may need to be modified for optimal image capture:
- For the non-Cyt-B method, perform a cell count using a commercially available cell counter following the manufacturer's instructions (see Table of Materials) on each culture immediately before culture and immediately after the recovery period.
- If running samples on a single camera imaging flow cytometer, place the Brightfield (BF) in Channel 4. Replace M01 with M04, and M07 with M01.
NOTE: "M" refers to the camera channel on the IFC. - Use the 40x magnification during acquisition.
- On the BF area versus BF aspect ratio plot during acquisition, use the following region coordinates:
X-coordinates: 100 and 900; Y-coordinates: 0.7 and 1 (Cyt-B method)
X-coordinates: 100 and 600; Y-coordinates: 0.7 and 1 - On the Hoechst intensity plot, use the following region coordinates:
X-coordinates: 55 and 75; Y-coordinates: 9.5 and 15 (Cyt-B method)
X-coordinates: 55 and 75; Y-coordinates: 13 and 21 (non-Cyt-B method) - To remove images of apoptotic and necrotic objects from the data, launch the IDEAS 6.3 software package (henceforth referred to as the "image analysis software"; see Table of Materials).
NOTE: The AI software has been designed to work with .daf files that have been processed using the latest version of the image analysis software. Ensure that the image analysis software is up to date. - Save this work as a template (.ast) file.
2. Creating .daf files for all .rif files
- The AI software only permits importing .daf files. Create .daf files for all .rif files in the experiment through batch processing.
- Under the Tools menu, click on Batch Data Files and then click on Add Batch.
- In the new window, select Add Files and select the .rif files to be added to the batch. Under the Select a Template or Data Analysis File (.ast, .daf) option, select the .ast file that was created previously.
- Assign a batch name if needed and click on OK to create .daf files for all loaded .rif files.
3. Creating an experiment in the AI software
- Refer to the flow chart in Figure 1 that describes the process of creating a deep learning model using the AI software.
- Launch the AI software and ensure the most recent version is installed by clicking on About in the bottom left corner of the window. If the most recent version is not installed, contact support@luminexcorp.com to obtain it.
- The default screen in the software is the New Experiment screen. Use the Folder icon to choose where to save the experiment, and type a name for the experiment (e.g., "MN model").
- Under Experiment Type, click the radio button beside Train to start a training experiment to begin building the CNN model. Click on Next.
- Optional: if a model has been previously trained, it can be used as a template for a new AI model, and can be selected as a template for creation of a new model from the Select Template Model screen. If no template model exists simply skip this step by clicking Next.
- The next screen is the Define New Model screen. Under Model, the name that was given to the model in step 3.3 will be automatically populated.
- Under Description, type a description for the model (optional) and leave the maximum image size at 150 pixels.
- Under Channels, click on Add BF to add a brightfield channel to the list. Under Name, double-click on Brightfield and rename this channel to BF. Click on Add FL to add a fluorescent channel to the list. Under Name, double-click on Fluorescent and rename this channel to DNA.
- Under Class Names, click on Add. In the pop-up window, type Mononucleated and click on OK. This adds the mononucleated class to the list of class names. Repeat this process to ensure the following six classes are defined in the list:
Mononucleated
Mononucleated with MN
Binucleated
Binucleated with MN
Polynucleated
Irregular morphology
Click on Next.
NOTE: These six ground truth model classes will represent the key events to be scored, as well as images with morphology that differs from the accepted scoring criteria5.- Optional: If desired, the analysis template from 1.7 can be included to use features from the image analysis software. If you would like to include these features in the AI model, browse for the .ast file, then from the channel specific dropdowns, choose the feature subsets you would like to include.
- Under Select Files, click on Add Files and browse for the desired files to be added to the AI software to build the ground truth data. Click on Next.
NOTE: It is important to add multiple data files (e.g., positive and negative control data) that contain a sufficient number of all key events.
- Next, on the Select Base Populations screen, locate the Non-Apoptotic population from the population hierarchy. Right-click on the Non-Apoptotic population and select Select All Matching Populations. Click on Next.
NOTE: It is important to exclude any populations that should not be classified (e.g., beads, debris, doublets, etc.) - This screen is the Select Truth Populations screen.
- If tagged truth populations of the key events have not been created in the image analysis software, then click on Next.
- If tagged truth populations have been created in the image analysis software, assign them to the appropriate model class.
- To assign a tagged truth population of MONO cells with MN, click on the Mononucleated with MN class under Model Classes on the left. Then click on the appropriate tagged truth population on the right that contains these events.
- If the tagged truth populations have been created in more than one data file, right-click on one of the truth populations and select Select All Matching Populations to add tagged populations from multiple files to the appropriate class.
- Once all appropriate truth populations have been assigned, click on Next.
- On the Select Channels screen, choose the appropriate channels for the experiment. Here, set BF to channel 1 and Hoechst to channel 7. Right-click on a channel and select Apply to All. Click on Next.
- Finally, on the Confirmation screen, click on Create Experiment.
- The AI software loads images from the data files and creates the model classes defined in step 3.5.3 with the ground truth imagery that was assigned in step 3.7. Click on Finish.
- Once the experiment is created, five options are presented:
Experiment: provides details of the experiment, including data files loaded, channels chosen, and defined ground truth model classes.
Tagging: launches the tagging tool through which users can populate ground truth data.
Training: trains a model based on the ground truth data.
Classify: uses trained models to classify data.
Results: provides results from both a training experiment and a classify experiment.
4. Populating the ground truth data using AI-assisted tagging tools
- Click on Tagging to launch the tagging tool interface.
- Click on the zoom tools (magnifying glass icons) to crop the images for easier viewing.
- Click on the slider bar to adjust the image size to change how many images are shown in the gallery.
- Click on the Display Setting option, and choose Min-Max, which provides the best contrast image for identifying all key events.
- Click on Setup Gallery Display to change the color of the DNA image to yellow or white, which will improve the visualization of small objects (e.g., MN).
- Click on Cluster to run the algorithm to group objects with similar morphology together. Once clustering is complete, the individual clusters with the number of objects per cluster are shown in a list under Unknown Populations. Select the individual clusters to view the objects within the cluster and assign these objects to their appropriate model classes.
- After a minimum of 25 objects have been assigned to each model class, the Predict algorithm becomes available. Click on Predict.
NOTE: Objects that don't fit well into any population remain classified as Unknown. As more objects are added to the truth populations, the prediction accuracy improves. - Continue to populate the ground truth model classes with appropriate imagery until a sufficient number of objects in each class is reached.
- Once a minimum of 100 objects have been assigned to each model class, click on the Training tab at the top of the screen. Click on the Train button to create a model using the Random Forest and CNN algorithms.
NOTE: The AI software creates models using both the Random Forest and CNN algorithms, the checkboxes permit creation of models using Random Forest of CNN algorithms only.
5. Assessing model accuracy
- Once model training is complete, click on View Results.
- Use the results screen to assess model accuracy. Use the pulldown menu to switch between Random Forest and CNN.
NOTE: The truth populations can be updated, and the model can be re-trained or used as-is to classify additional data.- To update the truth populations, click on Tagging at the top and follow section 4.
6. Classifying data using the model
- Launch the AI software. The default screen is the New Experiment screen. Use the Folder icon to choose where to save the experiment and type a name for the experiment.
- Under Experiment Type, click the radio button beside Classify to start a classification experiment. Click on Next.
- Click on the model to be used for classification, then click on Next.
- On the Select Files screen, click on Add Files and browse for the files to be classified by the CNN model. Click on Next.
- Next, on the Select Base Populations screen, click on the checkbox next to the Non-Apoptotic population in one of the loaded files. Right-click on the Non-Apoptotic population and click on Select All Matching Populations to select this population from all loaded files. Click on Next.
- Optional: if the data to be classified contains truth populations, they can be assigned to the appropriate model classes on the Select Truth Populations screen. Otherwise, click Next to skip this step.
- On the Select Channels screen, choose Channel 1 for brightfield and Channel 7 for the DNA stain. Right-click on a channel and click on Apply to All. Then click on Next.
- Finally, on the Confirmation screen, click on Create Experiment. The AI software loads the selected model and all images from the chosen data files. Click on Finish.
- Click on Classify to launch the classification screen. Click on the Classify button. This begins the process of using the RF and CNN model to classify additional data and identify all objects that belong in the specified model classes.
NOTE: The checkboxes can be used to select the RF model and/or the CNN model. - Once the classification is complete, click on View Results.
- Click the Update DAFs button to bring up the Update DAFs with Classification Results window. Click on OK to update the .daf files.
7. Generating a report of the classification results
- On the Results screen, click on Generate Report. Select the checkbox beside Create Report for Each Input DAF if an individual report for each input daf is required. Click on OK.
- Once completed, open the folder where the report files have been saved. Within the folder, there is an experiment .pdf report and a Resources folder.
- Open the .pdf to view the report. The report contains model and experiment information, the list of input .daf files, the class counts and class percentages in tabular and histogram format, and a confusion matrix summarizing the median prediction probability across all input .daf files.
- Open the Resources folder and then the CNN folder. Within this folder are .png files of the class count and percentage bar graphs, as well as the confusion matrix. Additionally, there are .csv files containing the class counts and percentages for each input file.
8. Determining MN frequency and cytotoxicity
- Calculating MN frequency
- Non Cyt-B method: To determine MN frequency, open the class_count.csv file from step 7.4. For each input file, divide the counts in the "Mononucleated with MN" population by the counts in the "Mononucleated" population and multiply by 100:

- Cyt-B method: To determine MN frequency, open the class_count.csv file from step 7.4. For each input file, divide the counts in the "Binucleated with MN" population by the counts in the "Binucleated" population and multiply by 100:
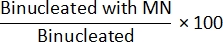
- Non Cyt-B method: To determine MN frequency, open the class_count.csv file from step 7.4. For each input file, divide the counts in the "Mononucleated with MN" population by the counts in the "Mononucleated" population and multiply by 100:
- Calculating cytotoxicity
- Non Cyt-B method:
- Using the initial cell counts and the post-treatment cell counts, first calculate the population doubling (PD) for each sample2:
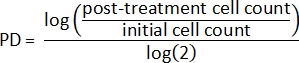
- Next, calculate the relative population doubling2:

- Finally, calculate the cytotoxicity2 for each sample:
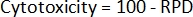
- Using the initial cell counts and the post-treatment cell counts, first calculate the population doubling (PD) for each sample2:
- Cyt-B method:
- To calculate Cytokinesis-Block Proliferation Index (CBPI)2, use the counts in the mononucleated, binucleated, and polynucleated classes for each sample from the class_count.csv file:
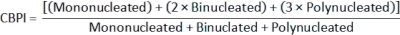
- To calculate cytotoxicity2, use the CBPI from the control cultures (C) and exposed cultures (T):
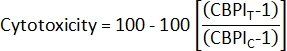
- To calculate Cytokinesis-Block Proliferation Index (CBPI)2, use the counts in the mononucleated, binucleated, and polynucleated classes for each sample from the class_count.csv file:
- Non Cyt-B method:
Results
Figure 1 shows the workflow for using the AI software to create a model for the MN assay. The user loads the desired .daf files into the AI software, then assigns objects to the ground truth model classes using the AI-assisted cluster (Figure 2) and predict (Figure 3) tagging algorithms. Once all ground truth model classes have been populated with sufficient objects, the model can be trained using the RF or CNN algorithms. Foll...
Discussion
The work presented here describes the use of deep learning algorithms to automate the scoring of the MN assay. Several recent publications have shown that intuitive, interactive tools allow the creation of deep learning models to analyze image data without the need for in-depth computational knowledge18,19. The protocol described in this work using a user interface-driven software package has been designed to work well with very large data files and permit the cr...
Disclosures
The authors are employed by Luminex Corporation, a DiaSorin Company, the manufacturer of the ImageStream imaging flow cytometer and the Amnis AI software used in this work.
Acknowledgements
None.
Materials
| Name | Company | Catalog Number | Comments |
| 15 mL centrifuge tube | Falcon | 352096 | |
| Cleanser - Coulter Clenz | Beckman Coulter | 8546931 | Fill container with 200 mL of Cleanser. https://www.beckmancoulter.com/wsrportal/page/itemDetails?itemNumber=8546931#2/10//0/25/ 1/0/asc/2/8546931///0/1//0/ |
| Colchicine | MilliporeSigma | 64-86-8 | |
| Corning bottle-top vacuum filter | MilliporeSigma | CLS430769 | 0.22 µm filter, 500 mL bottle |
| Cytochalasin B | MilliporeSigma | 14930-96-2 | 5 mg bottle |
| Debubbler - 70% Isopropanol | MilliporeSigma | 1.3704 | Fill container with 200 mL of Debubbler. http://www.emdmillipore.com/US/en/product/2-Propanol-70%25-%28V%2FV%29-0.1-%C2%B5m-filtred,MDA_CHEM-137040?ReferrerURL=https%3A%2F%2Fwww.google.com%2F |
| Dimethyl Sulfoxide (DMSO) | MilliporeSigma | 67-68-5 | |
| Dulbecco's Phosphate Buffered Saline 1X | EMD Millipore | BSS-1006-B | PBS Ca++MG++ Free |
| Fetal Bovine Serum | HyClone | SH30071.03 | |
| Formaldehyde, 10%, methanol free, Ultra Pure | Polysciences, Inc. | 04018 | This is what is used for the 4% and 1% Formalin. CAUTION: Formalin/Formaldehyde toxic by inhalation and if swallowed. Irritating to the eyes, respiratory systems and skin. May cause sensitization by inhalation or skin contact. Risk of serious damage to eyes. Potential cancer hazard. http://www.polysciences.com/default/catalog-products/life-sciences/histology-microscopy/fixatives/formaldehydes/formaldehyde-10-methanol-free-pure/ |
| Guava Muse Cell Analyzer | Luminex | 0500-3115 | A standard configuration Guava Muse Cell Analyzer was used. |
| Hoechst 33342 | Thermo Fisher | H3570 | 10 mg/mL solution |
| Mannitol | MilliporeSigma | 69-65-8 | |
| MEM Non-Essential Amino Acids 100X | HyClone | SH30238.01 | |
| MIFC - ImageStreamX Mark II | Luminex, a DiaSorin company | 100220 | A 2 camera ImageStreamX Mark II eqiped with the 405 nm, 488 nm, and 642 nm lasers was used. |
| MIFC analysis software - IDEAS | Luminex, a DiaSorin company | 100220 | "Image analysis sofware" The companion software to the MIFC (ImageStreamX MKII) |
| MIFC software - INSPIRE | Luminex, a DiaSorin company | 100220 | "Image acquisition software" This is the software that runs the MIFC (ImageStreamX MKII) |
| Amnis AI software | Luminex, a DiaSorin company | 100221 | "AI software" This is the software that permits the creation of artificial intelligence models to analyze data |
| Mitomycin C | MilliporeSigma | 50-07-7 | |
| NEAA Mixture 100x | Lonza BioWhittaker | 13-114E | |
| Penicllin/Streptomycin/Glutamine solution 100X | Gibco | 15070063 | |
| Potassium Chloride (KCl) | MilliporeSigma | P9541 | |
| Rinse - Ultrapure water or deionized water | NA | NA | Use any ultrapure water or deionized water. Fill container with 900 mL of Rinse. |
| RNase | MilliporeSigma | 9001-99-4 | |
| RPMI-1640 Medium 1x | HyClone | SH30027.01 | |
| Sheath - PBS | MilliporeSigma | BSS-1006-B | This is the same as Dulbecco's Phosphate Buffered Saline 1x Ca++MG++ free. Fill container with 900 mL of Sheath. |
| Sterile water | HyClone | SH30529.01 | |
| Sterilizer - 0.4%–0.7% Hypochlorite | VWR | JT9416-1 | This is assentually 10% Clorox bleach that can be made by deluting Clorox bleach with water. Fill container with 200 mL of Sterilzer. |
| T25 flask | Falcon | 353109 | |
| T75 flask | Falcon | 353136 | |
| TK6 cells | MilliporeSigma | 95111735 |
References
- Fenech, M., et al. HUMN project initiative and review of validation, quality control and prospects for further development of automated micronucleus assays using image cytometry systems. International Journal of Hygiene and Environmental Health. 216 (5), 541-552 (2013).
- OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test. Section 4. OECD Guidelines for the Testing of Chemicals. , (2016).
- Fenech, M. The in vitro micronucleus technique. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 455 (1), 81-95 (2000).
- Bonassi, S., et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis. 28 (3), 625-631 (2007).
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nature Protocols. 2 (5), 1084-1104 (2007).
- Fenech, M. Commentary on the SFTG international collaborative study on the in vitro micronucleus test: To Cyt-B or not to Cyt-B. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 607 (1), 9-12 (2006).
- Seager, A. L., et al. Recommendations, evaluation and validation of a semi-automated, fluorescent-based scoring protocol for micronucleus testing in human cells. Mutagenesis. 29 (3), 155-164 (2014).
- Rossnerova, A., Spatova, M., Schunck, C., Sram, R. J. Automated scoring of lymphocyte micronuclei by the MetaSystems Metafer image cytometry system and its application in studies of human mutagen sensitivity and biodosimetry of genotoxin exposure. Mutagenesis. 26 (1), 169-175 (2011).
- Bryce, S. M., Bemis, J. C., Avlasevich, S. L., Dertinger, S. D. In vitro micronucleus assay scored by flow cytometry provides a comprehensive evaluation of cytogenetic damage and cytotoxicity. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 630 (1), 78-91 (2007).
- Avlasevich, S. L., Bryce, S. M., Cairns, S. E., Dertinger, S. D. In vitro micronucleus scoring by flow cytometry: Differential staining of micronuclei versus apoptotic and necrotic chromatin enhances assay reliability. Environmental and Molecular Mutagenesis. 47 (1), 56-66 (2006).
- Basiji, D. A. Principles of Amnis imaging flow cytometry. Methods in Molecular Biology. 1389, 13-21 (2016).
- Rodrigues, M. A. Automation of the in vitro micronucleus assay using the Imagestream® imaging flow cytometer. Cytometry Part A. 93 (7), 706-726 (2018).
- Verma, J. R., et al. Investigating FlowSight® imaging flow cytometry as a platform to assess chemically induced micronuclei using human lymphoblastoid cells in vitro. Mutagenesis. 33 (4), 283-289 (2018).
- Wills, J. W., et al. Inter-laboratory automation of the in vitro micronucleus assay using imaging flow cytometry and deep learning. Archives of Toxicology. 95 (9), 3101-3115 (2021).
- Rodrigues, M. A., et al. The in vitro micronucleus assay using imaging flow cytometry and deep learning. Npj Systems Biology and Applications. 7 (1), 20 (2021).
- Rodrigues, M. A. An automated method to perform the in vitro micronucleus assay using multispectral imaging flow cytometry. Journal of Visualized Experiments. (147), e59324 (2019).
- Lovell, D. P., et al. Analysis of negative historical control group data from the in vitro micronucleus assay using TK6 cells. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 825, 40-50 (2018).
- Berg, S., et al. ilastik: interactive machine learning for (bio)image analysis. Nature Methods. 16 (12), 1226-1232 (2019).
- Hennig, H., et al. An open-source solution for advanced imaging flow cytometry data analysis using machine learning. Methods. 112, 201-210 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved