Summary
Abstract
Introduction
Protocol
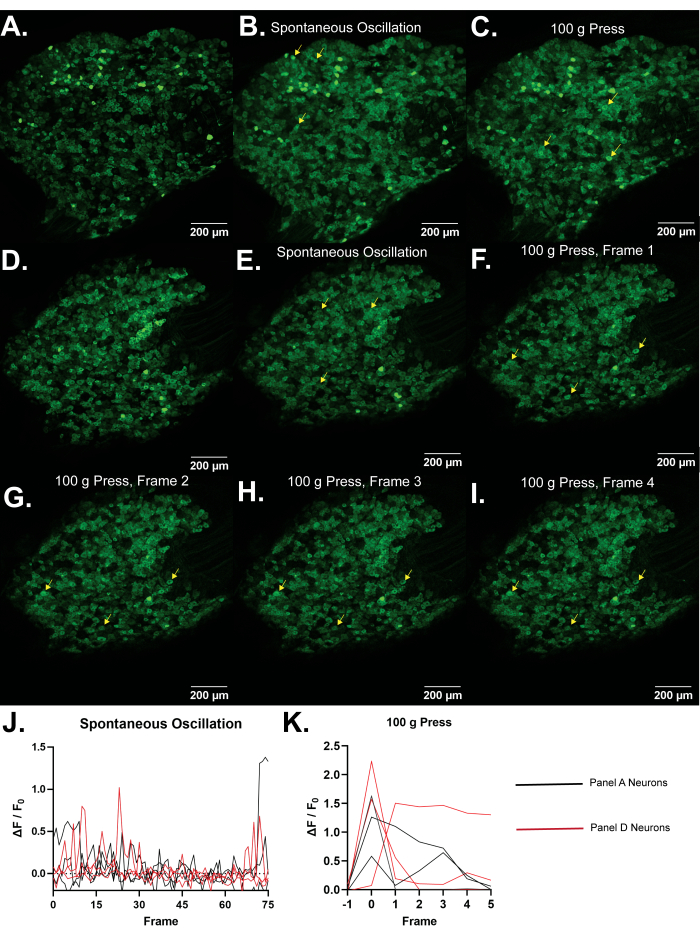
Representative Results
Discussion
Acknowledgements
Materials
References
Neuroscience
体内 完整背根神经节中初级感觉神经元网络中神经元集合体的钙成像
该协议描述了背根神经节(DRG)的手术暴露,然后是GCaMP3(基因编码的Ca2+ 指示剂;绿色荧光蛋白-钙调蛋白-M13蛋白3)使用Pirt-GCaMP3小鼠对神经元集合进行Ca2+ 成像,同时对同侧后爪施加各种刺激。
Ca 2+成像可用作细胞活动的代理,包括动作电位和涉及Ca 2+进入细胞质或释放细胞内Ca2+储存的各种信号机制。基于Pirt-GCaMP3的小鼠背根神经节(DRG)初级感觉神经元的Ca2+成像提供了同时测量大量细胞的优势。可以监测多达 1,800 个神经元,允许在体内种群水平的正常生理环境中将神经网络和体感过程作为一个整体进行研究。监测的大量神经元允许检测使用其他方法难以检测的活动模式。刺激可以应用于小鼠后爪,从而可以研究刺激对DRG神经元系综的直接影响。产生 Ca 2+ 瞬变的神经元数量以及 Ca2+ 瞬变的振幅表明对特定感觉模式的敏感性。神经元的直径提供了活化纤维类型的证据(非有害机械纤维与有害疼痛纤维,Aβ,Aδ和C纤维)。表达特定受体的神经元可以用td-Tomato和特异性Cre重组酶以及Pirt-GCaMP进行遗传标记。因此,DRG的Pirt-GCaMP3 Ca2+成像为分析特定的感觉模式和神经元亚型提供了一个强大的工具和模型,在人群水平上作为一个集合来研究疼痛,瘙痒,触摸和其他躯体感觉信号。
初级感觉神经元直接支配皮肤并将体感信息带回中枢神经系统1,2。背根神经节(DRGs)是由10,000-15,000个初级感觉神经元组成的细胞体簇3,4。DRG神经元表现出不同的大小、髓鞘形成水平以及基因和受体表达模式。较小直径的神经元包括疼痛感应神经元,较大直径的神经元通常对非疼痛的机械刺激做出反应5,6。初级感觉神经元的疾病,如损伤、慢性炎症和周围神经病,可以使这些神经元对各种刺激敏感,并导致慢性疼痛、异常性疼痛和疼痛超敏反应7,8。因此,DRG神经元的研究对于理解一般的躯体感觉和许多疼痛和瘙痒疾病都很重要。
体内放电的神经元对躯体感觉至关重要,但直到最近,研究体内完整神经节的工具仅限于相对较少的细胞数量9。在这里,我们描述了一种强大的方法,用于在体内集合中研究群体水平上神经元的动作电位或活动。该方法采用基于细....
| Name | Company | Catalog Number | Comments |
| Anased Injection (Xylazine) | Covetrus, Akorn | 33197 | |
| C Epiplan-Apochromat 10x/0.4 DIC | Cal Zeiss | 422642-9900-000 | |
| Cotton Tipped Applicators | McKesson | 24-106-1S | |
| Curved Hemostat | Fine Science Tools | 13007-12 | |
| DC Temperature Controller | FHC | 40-90-8D | |
| DC Temperature Controller Heating Pad | FHC | 40-90-2-05 | |
| Dumont Ceramic Coated Forceps | Fine Science Tools | 11252-50 | |
| FHC DC Temperature Controller | FHC | 40-90-8D | |
| Fluriso (Isoflurane) | MWI Animal Health, Piramal Group | 501017 | |
| Friedman-Pearson Rongeurs | Fine Science Tools | 16221-14 | |
| GelFoam | Pfizer | 09-0353-01 | |
| Ketaset (Ketamine) | Zoetis | KET-00002R2 | |
| Luminescent Green Stage Tape | JSITON/ Amazon | B803YW8ZWL | |
| Matrx VIP 3000 Isoflurane Vaporizer | Midmark | 91305430 | |
| Micro dissecting scissors | Roboz | RS-5882 | |
| Micro dissecting spring scissors | Fine Science Tools | 15023-10 | |
| Micro dissecting spring scissors | Roboz | RS-5677 | |
| Mini Rectal Thermistor Probe | FHC | 40-90-5D-02 | |
| Operating scissors | Roboz | RS-6812 | |
| Pirt-GCaMP3 C57BL/6J mice | Johns Hopkins University | N/A | Either sex can be imaged equally well. Mice should be at least 8 weeks old due to weak or intermittent Pirt promoter expression in younger mice. |
| SMALGO small animal algometer | Bioseb In vivo Research Instruments | BIO-SMALGO | |
| Stereotaxic frame | Kopf Model 923-B | 923-B | |
| td-Tomato C57BL/6J mice | Jackson Laboratory | 7909 | |
| Top Plate, 6 in x 10 in | Newport | 290-TP | |
| TrpV1-Cre C57BL/6J mice | Jackson Laboratory | 17769 | |
| Zeiss LSM 800 confocal microscope | Cal Zeiss | LSM800 | |
| Zeiss Zen 2.6 Blue Edition Software | Cal Zeiss | Zen (Blue Edition) 2.6 |
- Rivero-Melián, C., Grant, G. Distribution of lumbar dorsal root fibers in the lower thoracic and lumbosacral spinal cord of the rat studied with choleragenoid horseradish peroxidase conjugate. The Journal of Comparative Neurology. 299 (4), 470-481 (1990).
- Wessels, W. J., Marani, E. A rostrocaudal somatotopic organization in the brachial dorsal root ganglia of neonatal rats. Clinical Neurology and Neurosurgery. 95, 3-11 (1993).
- Schmalbruch, H. The number of neurons in dorsal root ganglia L4-L6 of the rat. The Anatomical Record. 219 (3), 315-322 (1987).
- Sørensen, B., Tandrup, T., Koltzenburg, M., Jakobsen, J. No further loss of dorsal root ganglion cells after axotomy in p75 neurotrophin receptor knockout mice. The Journal of Comparative Neurology. 459 (3), 242-250 (2003).
- Basbaum, A. I., Woolf, C. J. Pain. Current Biology. 9 (12), 429-431 (1999).
- Liu, Y., Ma, Q. Generation of somatic sensory neuron diversity and implications on sensory coding. Current Opinion in Neurobiology. 21 (1), 52-60 (2011).
- Basbaum, A. I., Bautista, D. M., Scherrer, G., Julius, D. Cellular and molecular mechanisms of pain. Cell. 139 (2), 267-284 (2009).
- Stucky, C. L., Mikesell, A. R. Cutaneous pain in disorders affecting peripheral nerves. Neuroscience Letters. 765, 136233 (2021).
- Iseppon, F., Linley, J. E., Wood, J. N. Calcium imaging for analgesic drug discovery. Neurobiology of Pain. 11, 100083 (2022).
- Chen, Z., et al. Adjacent intact nociceptive neurons drive the acute outburst of pain following peripheral axotomy. Scientific Reports. 9 (1), 7651 (2019).
- Chisholm, K. I., Khovanov, N., Lopes, D. M., La Russa, F., McMahon, S. B. Large scale in vivo recording of sensory neuron activity with GCaMP6. eNeuro. 5 (1), (2018).
- Emery, E. C., et al. In vivo characterization of distinct modality-specific subsets of somatosensory neurons using GCaMP. Science Advances. 2 (11), 1600990 (2016).
- Ishida, H., et al. In vivo calcium imaging visualizes incision-induced primary afferent sensitization and its amelioration by capsaicin pretreatment. The Journal of Neuroscience. 41 (41), 8494-8507 (2021).
- Kim, Y. S., et al. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron. 91 (5), 1085-1096 (2016).
- MacDonald, D. I., et al. Silent cold-sensing neurons contribute to cold allodynia in neuropathic pain. Brain. 144 (6), 1711-1726 (2021).
- Wang, F., et al. Sensory afferents use different coding strategies for heat and cold. Cell Reports. 23 (7), 2001-2013 (2018).
- Kucharczyk, M. W., et al. The impact of bone cancer on the peripheral encoding of mechanical pressure stimuli. Pain. 161 (8), 1894-1905 (2020).
- Kim, A. Y., et al. a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell. 133 (3), 475-485 (2008).
- Kim, Y. S., et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron. 81 (4), 873-887 (2014).
- Tian, L., et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods. 6 (12), 875-881 (2009).
- Thévenaz, P., Ruttimann, U. E., Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Transactions on Image Processing. 7 (1), 27-41 (1998).
- Mahadevan, A. S., et al. cytoNet: Spatiotemporal network analysis of cell communities. PLoS Computational Biology. 18 (6), 1009846 (2022).
- Barretto, R. P., et al. The neural representation of taste quality at the periphery. Nature. 517 (7534), 373-376 (2015).
- Leijon, S. C. M., et al. Oral thermosensing by murine trigeminal neurons: modulation by capsaicin, menthol and mustard oil. The Journal of Physiology. 597 (7), 2045-2061 (2019).
- Sekiguchi, K. J., et al. Imaging large-scale cellular activity in spinal cord of freely behaving mice. Nature Communications. 7, 11450 (2016).
- Wu, A., Dvoryanchikov, G., Pereira, E., Chaudhari, N., Roper, S. D. Breadth of tuning in taste afferent neurons varies with stimulus strength. Nature Communications. 6, 8171 (2015).
- Ran, C., Hoon, M. A., Chen, X. The coding of cutaneous temperature in the spinal cord. Nature Neuroscience. 19 (9), 1201-1209 (2016).
- Yarmolinsky, D. A., et al. Coding and plasticity in the mammalian thermosensory system. Neuron. 92 (5), 1079-1092 (2016).
- Torrance, N., Smith, B. H., Bennett, M. I., Lee, A. J. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. The Journal of Pain. 7 (4), 281-289 (2006).
- Shannonhouse, J., et al. Meclizine and metabotropic glutamate receptor agonists attenuate severe pain and Ca(2+) activity of primary sensory neurons in chemotherapy-induced peripheral neuropathy. The Journal of Neuroscience. 42 (31), 6020-6037 (2022).
- Luiz, A. P., et al. Cold sensing by Na(V)1.8-positive and Na(V)1.8-negative sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 116 (9), 3811-3816 (2019).
- Hartung, J. E., Gold, M. S. GCaMP as an indirect measure of electrical activity in rat trigeminal ganglion neurons. Cell Calcium. 89, 102225 (2020).
- Chung, M. K., Wang, S., Oh, S. L., Kim, Y. S. Acute and chronic pain from facial skin and oral mucosa: Unique neurobiology and challenging treatment. International Journal of Molecular Sciences. 22 (11), 5810 (2021).
- Chan, S. L., Mayne, M., Holden, C. P., Geiger, J. D., Mattson, M. P. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. The Journal of Biological Chemistry. 275 (24), 18195-18200 (2000).
- Sierra, D. A., Popov, S., Wilkie, T. M. Regulators of G-protein signaling in receptor complexes. Trends in Cardiovascular Medicine. 10 (6), 263-268 (2000).
- Yoshihara, K., et al. Astrocytic Ca(2+) responses in the spinal dorsal horn by noxious stimuli to the skin. Journal of Pharmacological Sciences. 137 (1), 101-104 (2018).
- Tan, C. H., McNaughton, P. A. The TRPM2 ion channel is required for sensitivity to warmth. Nature. 536 (7617), 460-463 (2016).
- Akemann, W., Mutoh, H., Perron, A., Rossier, J., Knöpfel, T. Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nature Methods. 7 (8), 643-649 (2010).
- Gong, Y., et al. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science. 350 (6266), 1361-1366 (2015).
- Grewe, B. F., Langer, D., Kasper, H., Kampa, B. M., Helmchen, F. High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision. Nature Methods. 7 (5), 399-405 (2010).
- Harada, K., et al. Red fluorescent protein-based cAMP indicator applicable to optogenetics and in vivo imaging. Scientific Reports. 7 (1), 7351 (2017).
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved