Summary
Abstract
Introduction
Protocol
Representative Results
Discussion
Acknowledgements
Materials
References
Neuroscience
In vivo Calciumbilleddannelse af neuronale ensembler i netværk af primære sensoriske neuroner i intakte dorsale rodganglier
Denne protokol beskriver den kirurgiske eksponering af dorsalrodsganglion (DRG) efterfulgt af GCaMP3 (genetisk kodet Ca2+ indikator; Grønt fluorescerende protein-calmodulin-M13-protein 3) Ca2+ billeddannelse af neuronale ensembler ved hjælp af Pirt-GCaMP3-mus, mens der påføres en række stimuli på den ipsilaterale bagpote.
Ca 2+ billeddannelse kan bruges som en proxy for cellulær aktivitet, herunder handlingspotentialer og forskellige signalmekanismer, der involverer Ca 2+ indgang i cytoplasmaet eller frigivelse af intracellulære Ca2+ lagre. Pirt-GCaMP3-baseret Ca2+ billeddannelse af primære sensoriske neuroner i dorsalrodsganglion (DRG) hos mus giver fordelen ved samtidig måling af et stort antal celler. Op til 1.800 neuroner kan overvåges, hvilket gør det muligt at studere neuronale netværk og somatosensoriske processer som et ensemble i deres normale fysiologiske kontekst på populationsniveau in vivo. Det store antal neuroner, der overvåges, gør det muligt at detektere aktivitetsmønstre, der ville være udfordrende at opdage ved hjælp af andre metoder. Stimuli kan påføres musebagpoten, hvilket gør det muligt at undersøge de direkte virkninger af stimuli på DRG-neuronensemblet. Antallet af neuroner, der producerer Ca 2+ transienter samt amplituden af Ca2+ transienter indikerer følsomhed over for specifikke sensoriske modaliteter. Diameteren af neuroner giver bevis for aktiverede fibertyper (ikke-skadelige mechano vs. skadelige smertefibre, Aβ-, Aδ- og C-fibre). Neuroner, der udtrykker specifikke receptorer, kan genetisk mærkes med td-Tomat og specifikke Cre-rekombinaser sammen med Pirt-GCaMP. Derfor giver Pirt-GCaMP3 Ca2+ billeddannelse af DRG et kraftfuldt værktøj og en model til analyse af specifikke sensoriske modaliteter og neuronundertyper, der fungerer som et ensemble på befolkningsniveau for at studere smerte, kløe, berøring og andre somatosensoriske signaler.
Primære sensoriske neuroner innerverer huden direkte og bærer somatosensorisk information tilbage til centralnervesystemet 1,2. Dorsalrodsganglier (DRG'er) er cellekropsklynger på 10.000-15.000 primære sensoriske neuroner 3,4. DRG-neuroner præsenterer forskellig størrelse, myelineringsniveauer og gen- og receptorekspressionsmønstre. Neuroner med mindre diameter inkluderer smertefølende neuroner, og neuroner med større diameter reagerer typisk på ikke-smertefulde mekaniske stimuli 5,6. Forstyrre....
Alle procedurer, der er beskrevet her, blev udført i overensstemmelse med en protokol, der er godkendt af Institutional Animal Care and Use Committee ved University of Texas Health Science Center i San Antonio.
BEMÆRK: Når dyrekirurgi (trin 1) og billeddannelse (trin 2) er påbegyndt, skal de gennemføres løbende. Dataanalyse (trin 3) kan udføres senere.
1. Kirurgi og sikring af dyret til højre side L5 DRG-billeddannelse
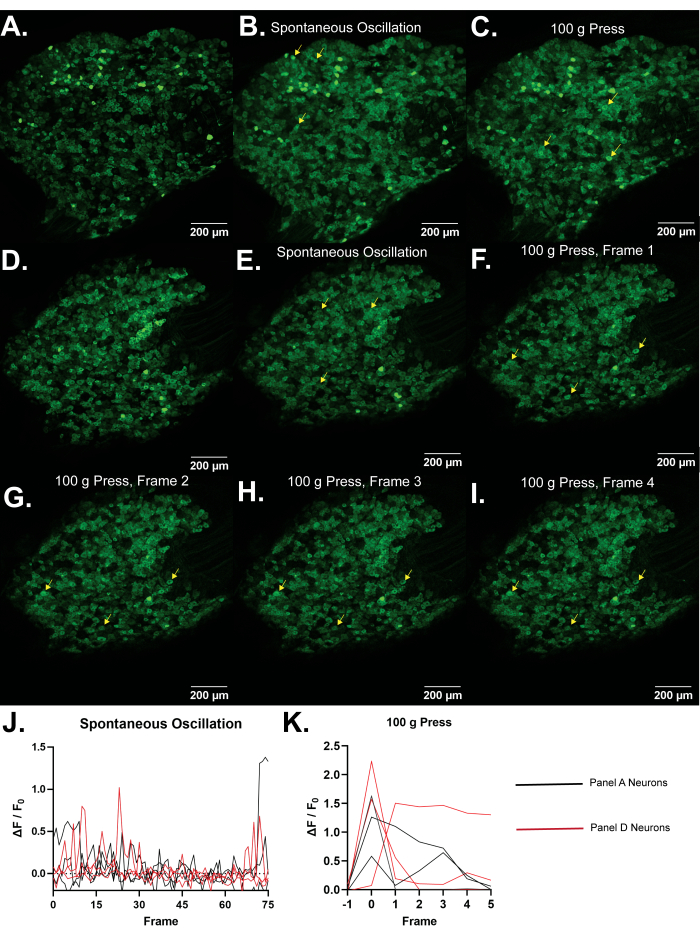
Figur 4: Repræsentative billeder af L5 dorsale rodganglier af Pirt-GCaMP3-mus. (A,D) Single frame højopløsningsscanninger af L5 dorsale rodganglier hos Pirt-GCaMP3-mus vises. (B,E) . Gennemsnitlige intensitetsfremskrivninger af 15 billeder af Pirt-GCaMP3 L5 DRG-ganglier fra henholdsvis panel A og.......
Vedvarende smerter er til stede i en lang række lidelser, invaliderende og / eller reducerer livskvaliteten for omkring 8% af mennesker29. Primære sensoriske neuroner registrerer skadelige stimuli på huden, og deres plasticitet bidrager til vedvarende smerte8. Mens neuroner kan studeres i cellekultur og eksplanter, fjerner det dem fra deres normale fysiologiske kontekst. Kirurgisk eksponering af DRG efterfulgt af Pirt-GCaMP3 Ca2+ billeddannelse tillader unders.......
Dette arbejde blev støttet af National Institutes of Health Grants R01DE026677 og R01DE031477 (til Y.S.K.), UTHSCSA startup fund (Y.S.K.) og en Rising Star Award fra University of Texas system (Y.S.K.).
....| Name | Company | Catalog Number | Comments |
| Anased Injection (Xylazine) | Covetrus, Akorn | 33197 | |
| C Epiplan-Apochromat 10x/0.4 DIC | Cal Zeiss | 422642-9900-000 | |
| Cotton Tipped Applicators | McKesson | 24-106-1S | |
| Curved Hemostat | Fine Science Tools | 13007-12 | |
| DC Temperature Controller | FHC | 40-90-8D | |
| DC Temperature Controller Heating Pad | FHC | 40-90-2-05 | |
| Dumont Ceramic Coated Forceps | Fine Science Tools | 11252-50 | |
| FHC DC Temperature Controller | FHC | 40-90-8D | |
| Fluriso (Isoflurane) | MWI Animal Health, Piramal Group | 501017 | |
| Friedman-Pearson Rongeurs | Fine Science Tools | 16221-14 | |
| GelFoam | Pfizer | 09-0353-01 | |
| Ketaset (Ketamine) | Zoetis | KET-00002R2 | |
| Luminescent Green Stage Tape | JSITON/ Amazon | B803YW8ZWL | |
| Matrx VIP 3000 Isoflurane Vaporizer | Midmark | 91305430 | |
| Micro dissecting scissors | Roboz | RS-5882 | |
| Micro dissecting spring scissors | Fine Science Tools | 15023-10 | |
| Micro dissecting spring scissors | Roboz | RS-5677 | |
| Mini Rectal Thermistor Probe | FHC | 40-90-5D-02 | |
| Operating scissors | Roboz | RS-6812 | |
| Pirt-GCaMP3 C57BL/6J mice | Johns Hopkins University | N/A | Either sex can be imaged equally well. Mice should be at least 8 weeks old due to weak or intermittent Pirt promoter expression in younger mice. |
| SMALGO small animal algometer | Bioseb In vivo Research Instruments | BIO-SMALGO | |
| Stereotaxic frame | Kopf Model 923-B | 923-B | |
| td-Tomato C57BL/6J mice | Jackson Laboratory | 7909 | |
| Top Plate, 6 in x 10 in | Newport | 290-TP | |
| TrpV1-Cre C57BL/6J mice | Jackson Laboratory | 17769 | |
| Zeiss LSM 800 confocal microscope | Cal Zeiss | LSM800 | |
| Zeiss Zen 2.6 Blue Edition Software | Cal Zeiss | Zen (Blue Edition) 2.6 |
- Rivero-Melián, C., Grant, G. Distribution of lumbar dorsal root fibers in the lower thoracic and lumbosacral spinal cord of the rat studied with choleragenoid horseradish peroxidase conjugate. The Journal of Comparative Neurology. 299 (4), 470-481 (1990).
- Wessels, W. J., Marani, E. A rostrocaudal somatotopic organization in the brachial dorsal root ganglia of neonatal rats. Clinical Neurology and Neurosurgery. 95, 3-11 (1993).
- Schmalbruch, H. The number of neurons in dorsal root ganglia L4-L6 of the rat. The Anatomical Record. 219 (3), 315-322 (1987).
- Sørensen, B., Tandrup, T., Koltzenburg, M., Jakobsen, J. No further loss of dorsal root ganglion cells after axotomy in p75 neurotrophin receptor knockout mice. The Journal of Comparative Neurology. 459 (3), 242-250 (2003).
- Basbaum, A. I., Woolf, C. J. Pain. Current Biology. 9 (12), 429-431 (1999).
- Liu, Y., Ma, Q. Generation of somatic sensory neuron diversity and implications on sensory coding. Current Opinion in Neurobiology. 21 (1), 52-60 (2011).
- Basbaum, A. I., Bautista, D. M., Scherrer, G., Julius, D. Cellular and molecular mechanisms of pain. Cell. 139 (2), 267-284 (2009).
- Stucky, C. L., Mikesell, A. R. Cutaneous pain in disorders affecting peripheral nerves. Neuroscience Letters. 765, 136233 (2021).
- Iseppon, F., Linley, J. E., Wood, J. N. Calcium imaging for analgesic drug discovery. Neurobiology of Pain. 11, 100083 (2022).
- Chen, Z., et al. Adjacent intact nociceptive neurons drive the acute outburst of pain following peripheral axotomy. Scientific Reports. 9 (1), 7651 (2019).
- Chisholm, K. I., Khovanov, N., Lopes, D. M., La Russa, F., McMahon, S. B. Large scale in vivo recording of sensory neuron activity with GCaMP6. eNeuro. 5 (1), (2018).
- Emery, E. C., et al. In vivo characterization of distinct modality-specific subsets of somatosensory neurons using GCaMP. Science Advances. 2 (11), 1600990 (2016).
- Ishida, H., et al. In vivo calcium imaging visualizes incision-induced primary afferent sensitization and its amelioration by capsaicin pretreatment. The Journal of Neuroscience. 41 (41), 8494-8507 (2021).
- Kim, Y. S., et al. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron. 91 (5), 1085-1096 (2016).
- MacDonald, D. I., et al. Silent cold-sensing neurons contribute to cold allodynia in neuropathic pain. Brain. 144 (6), 1711-1726 (2021).
- Wang, F., et al. Sensory afferents use different coding strategies for heat and cold. Cell Reports. 23 (7), 2001-2013 (2018).
- Kucharczyk, M. W., et al. The impact of bone cancer on the peripheral encoding of mechanical pressure stimuli. Pain. 161 (8), 1894-1905 (2020).
- Kim, A. Y., et al. a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell. 133 (3), 475-485 (2008).
- Kim, Y. S., et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron. 81 (4), 873-887 (2014).
- Tian, L., et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods. 6 (12), 875-881 (2009).
- Thévenaz, P., Ruttimann, U. E., Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Transactions on Image Processing. 7 (1), 27-41 (1998).
- Mahadevan, A. S., et al. cytoNet: Spatiotemporal network analysis of cell communities. PLoS Computational Biology. 18 (6), 1009846 (2022).
- Barretto, R. P., et al. The neural representation of taste quality at the periphery. Nature. 517 (7534), 373-376 (2015).
- Leijon, S. C. M., et al. Oral thermosensing by murine trigeminal neurons: modulation by capsaicin, menthol and mustard oil. The Journal of Physiology. 597 (7), 2045-2061 (2019).
- Sekiguchi, K. J., et al. Imaging large-scale cellular activity in spinal cord of freely behaving mice. Nature Communications. 7, 11450 (2016).
- Wu, A., Dvoryanchikov, G., Pereira, E., Chaudhari, N., Roper, S. D. Breadth of tuning in taste afferent neurons varies with stimulus strength. Nature Communications. 6, 8171 (2015).
- Ran, C., Hoon, M. A., Chen, X. The coding of cutaneous temperature in the spinal cord. Nature Neuroscience. 19 (9), 1201-1209 (2016).
- Yarmolinsky, D. A., et al. Coding and plasticity in the mammalian thermosensory system. Neuron. 92 (5), 1079-1092 (2016).
- Torrance, N., Smith, B. H., Bennett, M. I., Lee, A. J. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. The Journal of Pain. 7 (4), 281-289 (2006).
- Shannonhouse, J., et al. Meclizine and metabotropic glutamate receptor agonists attenuate severe pain and Ca(2+) activity of primary sensory neurons in chemotherapy-induced peripheral neuropathy. The Journal of Neuroscience. 42 (31), 6020-6037 (2022).
- Luiz, A. P., et al. Cold sensing by Na(V)1.8-positive and Na(V)1.8-negative sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 116 (9), 3811-3816 (2019).
- Hartung, J. E., Gold, M. S. GCaMP as an indirect measure of electrical activity in rat trigeminal ganglion neurons. Cell Calcium. 89, 102225 (2020).
- Chung, M. K., Wang, S., Oh, S. L., Kim, Y. S. Acute and chronic pain from facial skin and oral mucosa: Unique neurobiology and challenging treatment. International Journal of Molecular Sciences. 22 (11), 5810 (2021).
- Chan, S. L., Mayne, M., Holden, C. P., Geiger, J. D., Mattson, M. P. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. The Journal of Biological Chemistry. 275 (24), 18195-18200 (2000).
- Sierra, D. A., Popov, S., Wilkie, T. M. Regulators of G-protein signaling in receptor complexes. Trends in Cardiovascular Medicine. 10 (6), 263-268 (2000).
- Yoshihara, K., et al. Astrocytic Ca(2+) responses in the spinal dorsal horn by noxious stimuli to the skin. Journal of Pharmacological Sciences. 137 (1), 101-104 (2018).
- Tan, C. H., McNaughton, P. A. The TRPM2 ion channel is required for sensitivity to warmth. Nature. 536 (7617), 460-463 (2016).
- Akemann, W., Mutoh, H., Perron, A., Rossier, J., Knöpfel, T. Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nature Methods. 7 (8), 643-649 (2010).
- Gong, Y., et al. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science. 350 (6266), 1361-1366 (2015).
- Grewe, B. F., Langer, D., Kasper, H., Kampa, B. M., Helmchen, F. High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision. Nature Methods. 7 (5), 399-405 (2010).
- Harada, K., et al. Red fluorescent protein-based cAMP indicator applicable to optogenetics and in vivo imaging. Scientific Reports. 7 (1), 7351 (2017).
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved