Ex Vivo Perfusion Culture of Large Blood Vessels in a 3D Printed Bioreactor
* These authors contributed equally
In This Article
Summary
This protocol presents the setup and operation of a newly developed, 3D printed bioreactor for the ex vivo culture of blood vessels in perfusion. The system is designed to be easily adopted by other users, practical, affordable, and adaptable to different experimental applications, such as basic biology and pharmacological studies.
Abstract
Vascular disease forms the basis of most cardiovascular diseases (CVDs), which remain the primary cause of mortality and morbidity worldwide. Efficacious surgical and pharmacological interventions to prevent and treat vascular disease are urgently needed. In part, the shortage of translational models limits the understanding of the cellular and molecular processes involved in vascular disease. Ex vivo perfusion culture bioreactors provide an ideal platform for the study of large animal vessels (including humans) in a controlled dynamic environment, combining the ease of in vitro culture and the complexity of the live tissue. Most bioreactors are, however, custom manufactured and therefore difficult to adopt, limiting the reproducibility of the results. This paper presents a 3D printed system that can be easily produced and applied in any biological lab, and provides a detailed protocol for its setup, enabling users' operation. This innovative and reproducible ex vivo perfusion culture system enables the culture of blood vessels for up to 7 days in physiological conditions. We expect that adopting a standardized perfusion bioreactor will support a better understanding of physiological and pathological processes in large animal blood vessels and accelerate the discovery of new therapeutics.
Introduction
The vascular wall exists in a reactive steady state, which ensures both responsivities to external stimuli (i.e., change of pressure, vasoconstrictors) and a consistent non-activating surface preventing blood coagulation and inflammatory cell infiltration1. In response to aging- and lifestyle-dependent stimuli and upon direct damage, the vascular wall activates remodeling processes such as restenosis and atherosclerosis, which are known contributors to common cardiovascular diseases (CVDs), such as ischemic stroke and myocardial infarction2. While interventional approaches such as percutaneous revascularisation and stenting are available to tackle advanced manifestations of vascular disease, these are known to provoke further vascular damage, often leading to recurrence. In addition, only limited preventative and early-stage solutions are available. Understanding the mechanisms maintaining vascular wall homeostasis and driving its dysfunction is at the heart of developing new cures3.
Despite the constant development and advances in molecular biology and tissue engineering, animal studies remain a crucial component of vascular biology studies. In vivo animal studies have provided enormous insight into the mechanisms of vascular homeostasis and pathology; however, these procedures are costly, have relatively low throughput, and pose substantial ethical issues. In addition, small animals are poorly representative of human vascular physiology, and larger animal experiments are vastly more expensive and create further ethical considerations4,5. With the increasing demand for pharmaceutical and medical solutions for a rapidly aging population, the downsides of animal use are magnified, impacting the reproducibility, reliability, and transferability of results to patient care6.
In vitro systems offer a simplified platform to study basic mechanisms but fail to recapitulate the complexity of the whole tissue, the interactions between cells and the extracellular matrix, and the mechanical forces, which are critical determinants in the development of vascular diseases7.
Ex vivo studies performed on whole tissues maintained in artificially controlled environments mimic the in vivo complexity while enabling relatively high-throughput investigations8. Given the ability to closely control the culture conditions and environment, ex vivo models allow a broad range of complex studies and provide a suitable alternative to reduce the use of animal procedures in vascular biology. Static vascular ring cultures offered interesting insights but failed to incorporate the crucial hemodynamic element9. Indeed, the study of the vascular system ex vivo poses specific challenges related to the many dynamic forces which apply to the cells within the blood vessel wall. Stimuli such as luminal flow, turbulence, shear stress, pressure, and wall deformation significantly impact tissue pathophysiology10,11,12.
Perfusion bioreactors are essential for studying vascular homeostasis and remodeling in response to injury or hemodynamic changes13. Furthermore, perfusion culture can be used to improve the maturation and durability of tissue-engineered blood vessels (TEBVs), providing suitable alternatives for vascular grafts14.
Commercially available perfusion bioreactors are limited in flexibility and adaptability and are costly. Many of the existing in-house developed bioreactors are instead difficult to replicate in other labs, due to the limited descriptions and unavailability of specially made components7,8,9,10,11,12. To overcome these limitations, we have recently developed a new bioreactor (EasyFlow), which is economical to produce, accommodates a range of tissues, and enables relatively simple modifications to adapt to different research demands13. The insert is 3D printed and fits as in a lid of a standard 50 mL centrifuge tube. Its modular design and 3D printing manufacture make it accessible and reproducible across different labs, as well as easily modifiable to adapt to different scientific needs. This protocol describes the assembly and basic operation of the bioreactor system in an arterial perfusion setting.
Protocol
This protocol describes the assembly and use of a system composed of two EasyFlow (bioreactor) inserts: one representing the reaction chamber (C), containing the perfused artery sample, and one functioning as a medium reservoir (R) (Figure 1 and Figure 2A). Carotid arteries were obtained from 4-6-week-old male and female piglets (6-12 kg) at The Pirbright Institute, UK. Animal procedures were carried out under the Home Office Animals (Scientific Procedures) Act (1986) (ASPA) and approved by the Animal Welfare and Ethical Review Board (AWERB) at The Pirbright Institute. The animals were housed in accordance with the Code of Practice for the Housing and Care of Animals Bred. All procedures were conducted by Personal License holders who were trained and competent under Project License PPL70/8852. Piglets were culled according to the schedule one method under the ASPA.
1. Insert manufacturing
- Fabricate the insert by 3D printing, using the provided 3D model (Supplementary File 1).
NOTE: 3D modeling enables easy changes to the design to adapt to new applications. Alternative materials and alternative manufacturing techniques can also be used to produce the insert. Due to the complex inner structure, selective laser sintering and stereolithography are appropriate alternatives15. Polyamide 12 (PA12; see Table of Materials) is a good material candidate due to its superior performance in terms of liquid retention and resistance to repeated heat sterilization cycles16. - Fabricate the silicon gaskets and polycarbonate washers by laser cutting17, using the designs provided in Supplementary File 2.
NOTE: Laser cutting is easily outsourced and a cheap manufacturing method. Washers can be manufactured from stainless steel, providing a more resistant component for repeated use. All other components are commercially available items. A complete list of the required materials is provided in Table 1. The commercial details of the items are included in the Table of Materials.
2. Device sterilization, assembly, and priming
- Sterilize all components following the instructions in Table 1.
- Under laminar flow conditions, assemble two fabricated inserts (step 1), as shown in Figure 1A.
- Assemble the perfusion system as in Figure 2, following the steps below:
- Attach two connected three-way valves to the reservoir's R1 port (media exchange port).
- Connect the resulting outlet to the peristaltic pump's head using a tube equipped with a one-way valve (one-way valve tube).
- Connect the pump head to the reaction chamber's C4 port using system tubing equipped with a one-way valve.
NOTE: This branch can optionally be equipped with a pressure sensor enabling constant monitoring. - Connect the reaction chamber port C1 to soft wall tubing, and this to the resistance channel (small inner diameter).
NOTE: The length of the resistance tubing will greatly influence the pressure present in the system. This should be further investigated to ensure adequate perfusion conditions. - Extend the resistance channel with the system tubing, creating a return channel and closing the luminal circulation loop by connecting to the reservoir at R5 (Figure 2C).
- Create an overflow channel by connecting the reaction chamber C3 to the reservoir R3 with system tubing.
- Attach a ventilation filter through R2.
- Create a pressure dampener by connecting a syringe containing air and media to the reaction chamber C6.
NOTE: The correct air-to-media ratio will depend on the required pressure dampening.
- Prime the system with perfusion medium (Dulbecco's modified eagle medium [DMEM] + 10% [v/v] fetal bovine serum [FBS] + 1% [v/v] penicillin-streptomycin + 1% [v/v] amphotericin B + 30% [v/v] dextran; see Table of Materials) through the media exchange ports and the reservoir.
NOTE: Priming the system reduces the risk of trapped bubbles in the system and identifies any potential leaks. The recommended volume of media is about 100-120 mL. The volume used will depend on the dead volume of the tubing used for experimentation.
3. Sample harvesting and preparation
- Collect the left and right common carotid arteries, minimizing direct handling of the arterial tissue13.
- Place the tissue in cold transport media (DMEM+ 20% [v/v] FBS + 2% [v/v] penicillin-streptomycin + 1% [v/v] amphotericin B, see Table of Materials) for transfer.
- In a laminar flow cabinet, remove the excess connective tissue and trim the ends of the tissue using a scalpel blade. Wash the tissue twice in cold transport media.
- Place tissue on an orbital shaker in transport media for at least 30 min for thorough washing.
- Connect a non-branching segment of the artery to the bioreactor system using two barbed luer connectors and fix it in place using a vessel bond (vascular silicone ties; Table 1).
NOTE: The vessel bond provides the proper tension and retraction to secure the vessel. The oval section prevents tissue damage. - Gently flow media through the artery to verify patency.
- After securing the artery to the fabricated insert, fill the reaction space with perfusion media (step 2.4). Finally, gently fill the luminal circulation loop with media to eliminate any remaining air from the system.
- Connect the reaction space with the previously assembled perfusion system (section 2), completing the circulation.
NOTE: It is recommended to perform a quality check by immunohistochemistry of processed tissue. This identifies any damage due to excessive handling during preparation.
4. Perfusion culture and media change
- Place the perfusion system in a 37 °C incubator with 5% CO2 and then connect it to a peristaltic pump (see Table of Materials)
- Connect any additional (facultative) acquisition systems, such as pressure sensors (see Table of Materials).
- Leave the system to equilibrate overnight with a low media flow rate of ~10-15 mL/min.
NOTE: Initial experiments to determine pump settings, media flow, system pressure, and optimal pressure dampeners/clamps settings are required to ensure appropriate conditions are met. - The next day, increase the flow incrementally (+1 rpm every hour, equivalent to a ~2.5 mL/min increase every hour, in the current system) until the final flow rate (35 mL/min) is achieved. Monitor the system periodically for leaks.
NOTE: To calculate the accurate flowrate based on the peristaltic pump speed, users must first perform adequate pump calibration18. Using the formula (1), volumetric flow rate (Q) can be calculated based on the volume dispensed (V) within a given time (t). To calculate the estimated flow velocity (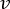 ) we can use the previously calculated flow rate (Q) and the area of vessel cross-section (A), described in equation (2).
) we can use the previously calculated flow rate (Q) and the area of vessel cross-section (A), described in equation (2).
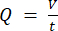 (1)
(1)
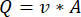 (2)
(2) - Every 3 days, exchange 50% of the media (~50 mL) by connecting one syringe filled with fresh media to the media exchange port closer to the pump and an empty syringe to the port closer to the reservoir to collect the spent medium (Figure 2).
NOTE: Using two three-way valves as media exchange ports facilitates the continuous operation of the perfusion during the medium exchange. - At the end of the experiment, harvest the tissue from the reaction chamber by trimming the ends connected to the bioreactor using sterile surgical scissors.
- Dismantle, clean, and sterilize the system for further use.
5. Sample analysis
- Fix the harvested sample in 4% paraformaldehyde (PFA) overnight at 4 °C.
- Embed the tissue in optimal cutting temperature (OCT; see Table of Materials)19 and freeze by immersion in iso-pentane cooled in liquid nitrogen.
- Obtain cryo-sections of 3-5 mm thickness using a cryostat.
- Perform immunohistochemistry (haematoxylin and eosin [H&E]) and/or immunofluorescence. Follow the typical immunofluorescence protocol:
- Block the slices for 1 h at room temperature with 20% [v/v] goat serum in phosphate-buffered saline (PBS; see Table of Materials).
- Incubate the slices overnight at 4 °C with primary rabbit antibody against CD31 diluted 1:50 in PBS (see Table of Materials).
- Wash three times with PBS for 5 min.
- Incubate for 1 h at 37 °C with fluorescently labeled goat anti-rabbit secondary antibody diluted 1:200 in PBS and α-smooth muscle actin (SMA) directly conjugated fluorescent antibody diluted 1:200 in PBS (see Table of Materials).
- Wash three times with PBS for 5 min.
- Stain the nuclei with DAPI diluted 1:1,000 in PBS for 10 min at room temperature.
- Incubate with 0.1% (w/v) Sudan Black (see Table of Materials) in 70% (v/v) ethanol for 10 min at room temperature to reduce tissue autofluorescence.
- Wash the tissue with abundant deionized water. Mount the slides in the mounting medium.
- Image the samples with a confocal laser scanning microscope.
Representative Results
This study has established a versatile and affordable perfusion system (EasyFlow)13. The 3D printed design of the system facilitates the adoption of the system by other labs and therefore encourages reproducibility.
The fabricated perfusion insert is housed in a 50 mL centrifuge tube, creating an isolated environment. Using two perfusion inserts, a perfusion loop can be established containing a reservoir and a reaction chamber, where the biological sample is incubated. The perfusion system is then connected to a peristaltic pump and optional acquisition systems, such as pressure sensors and ultrasound devices, to monitor culture conditions (Figure 2).
Porcine carotid artery samples were collected and processed before being cultured in perfusion for 7 days. To ensure the quality of the tissue before culture, preliminary experiments were performed where the samples were fixed at the time of excision, after tissue preparation, and after perfusion. Fluorescent staining for endothelial (CD31) and smooth muscle (αSMA) markers was used to assess the maintenance of tissue integrity. Examples of well-preserved and damaged tissues are presented in Figure 3 for comparison. The images show the importance of gentle handling of the tissue at the time of excision, as incorrect processing (excessive stretch, crushing, etc.) may result in loss of the endothelium ahead of the culture. Results also show the importance of the gradual establishment of perfusion to avoid luminal damage.
H&E staining was performed alongside immunofluorescence (IF) staining to show maintenance of the morphology and overall distribution of the cells in the vessel wall after 7 days of culture (Figure 4). The application of physiological culture conditions in the device ensures maintenance of the endothelial coverage of the lumen, alignment of the smooth muscle cells in the media, and preservation of the vasa vasorum in the adventitia.
The compact and modular design of the bioreactor system also allows for a wide range of system setups. The smaller setup comprises a single bioreactor and is ideal for pharmacological and low-volume studies (recirculating perfusion20, total volume of 50-70 mL). To increase the length of culture and reduce the number of media changes, a single reservoir circulation system, like the one described in this protocol, or a constant feeding system is more ideal, as they have a larger volume of medium in circulation. A double circulation21 setup can be established to explore experimental settings where the localization of the stimuli is critical. The current device can also be combined with more sophisticated control systems to enable precise feedback control of parameters such as pH and dissolved oxygen (Figure 5).
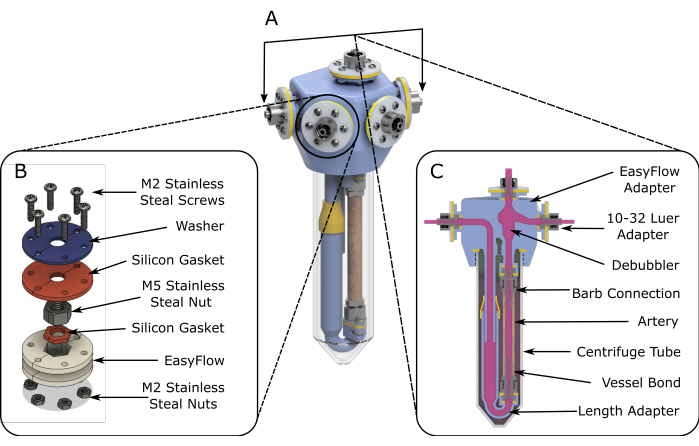
Figure 1: EasyFlow assembly schematics. (A) 3D rendered schematics aiding the assembly of the perfusion insert. (B) Detailed schematics are provided to facilitate assembly of the connection sites. (C) A cross-section view of the reaction space highlights the essential components of the EasyFlow insert and the connection of the tissue to the insert. Please click here to view a larger version of this figure.
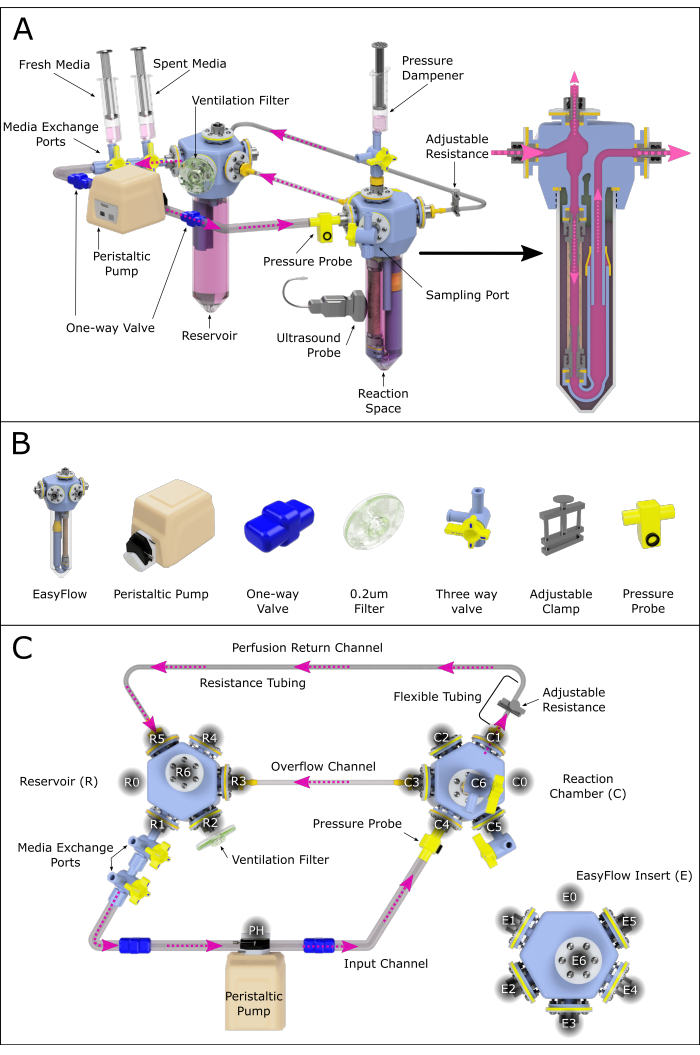
Figure 2: Schematic representation of the perfusion system assembly. (A) Assembled perfusion system containing all important components, highlighting their relative position in a 3D-rendered environment. Not all the components are to scale. (B) Individual isometric views of components are also presented. (C) A top view of the assembled perfusion system is shown to aid the assembly and connection of the different components. The ports have been labeled and numbered counterclockwise to navigate the various connection sites across the perfusion system. This principle has been applied to the reservoir (R) and reaction chamber (C). The various channels connecting the two chambers have also been assigned names for reference. Please click here to view a larger version of this figure.
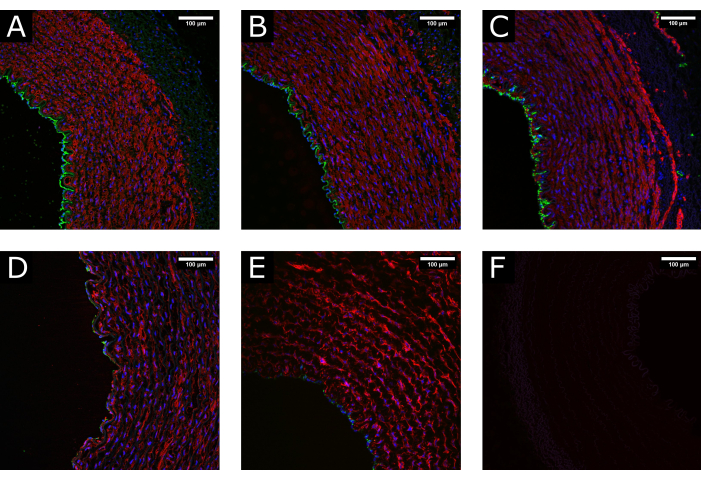
Figure 3: Tissue maintenance during processing. (A) Confocal images show the normal structure of an artery at the time of harvest, (B) after cleaning and processing, and (C) after perfusion culture, demonstrating the maintenance of a well-preserved morphology. (D) Examples of damaged tissue due to excessive or incorrect handling during processing or (E) due to the application of non-physiological culture conditions (i.e., abrupt initiation of high flow) show depletion of the luminal coverage and disruption of the media. (F) Negative control indicates the specificity of the staining. CD31: green; αSMA: red; DAPI: blue. Scale bars: 100 µm. Please click here to view a larger version of this figure.
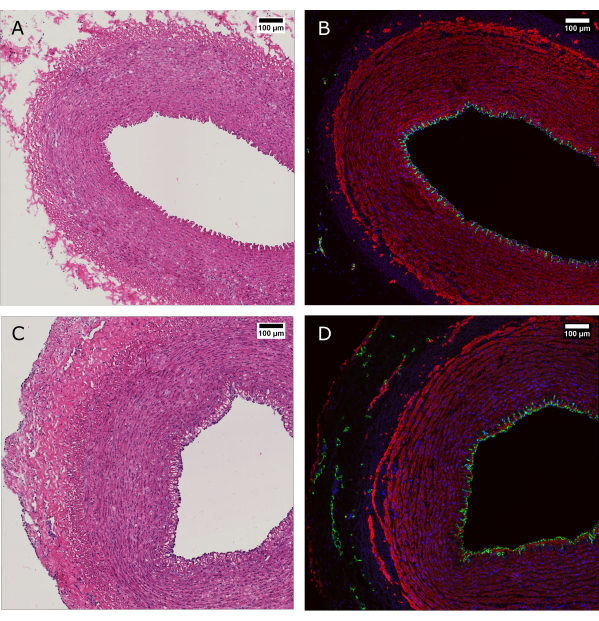
Figure 4: Histological evaluation of tissue before and after perfusion culture. (A and B) Arterial tissue was evaluated by histology at the time of harvest and (C and D) after 7 days of culture with the bioreactor system. (A and C) H&E staining reveals preservation of the arterial wall structure and organization. (B and D) Immunofluorescence staining of the same tissue evidence endothelial coverage, smooth muscle cell alignment, and vasa vasorum in the adventitia. CD31: green; SMA: red; DAPI: blue. Scale bars: 100 µm. Please click here to view a larger version of this figure.
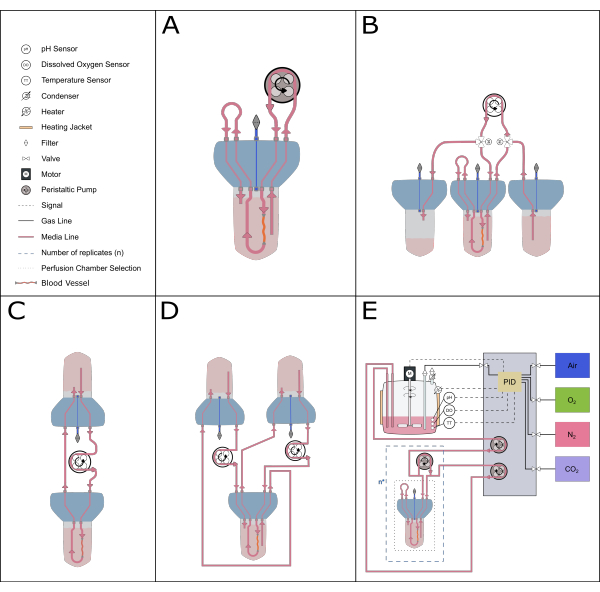
Figure 5: Schematic representation of possible perfusion setups. Various alternative setups can be accommodated with the perfusion insert to enable different experimental studies. (A) The recirculating perfusion setup minimizes the volume of medium required. (B) The constant feeding setup provides a steady supply of medium to the tissue. (C) Single reservoir circulation (as described in this paper) provides a larger volume of media for long-term incubations and includes a buffer zone for air exchange and pressure equilibration. (D) The double circulation setup provides two distinct loops feeding the inner (inside the artery) and outer (reaction space) circulations independently. (E) The dynamic perfusion setup includes continuous gasses and pH control by a proportional-integral-derivative (PID) controller. Please click here to view a larger version of this figure.
Table 1: Components for fabricating the inserts. Please click here to download this Table.
Supplementary File 1: 3D model of the EasyFlow insert for 3D printing manufacture. Please click here to download this File.
Supplementary File 2: Design schematics of the constructed device, section view, printout, and sealing components. Please click here to download this File.
Discussion
Ex vivo vascular perfusion systems constitute a unique platform to study the function and behavior of vascular cells within their native tissues under controlled conditions, which enables the dissection of complex processes such as post-injury vascular remodeling22. However, most reported bioreactors are in-house made systems based on custom-made components and are often difficult to replicate by others23. Alternative commercial solutions exist, but lack flexibility in the design and can be relatively costly24.
We have developed an alternative system that provides an easy, cheap, and reproducible platform that can be manufactured using open-source 3D printing techniques13. The present article describes the system's setup to enable reproducible applications by end-users. This setup enables the application of physiological and pathological conditions of pressure (40-180 mmHg), flow rate (6-30 mL/min), and in combination with media mimicking the blood viscosity, with varying degrees of shear stress.
Reproducibility is an essential aspect of the scientific process, as it allows researchers to validate the findings of others and build upon them to progress our understanding of vascular diseases. Furthermore, tools that enable and promote collaborations between groups are essential for advancing scientific knowledge. EasyFlow represents an example of such open-source, accessible solutions that can be easily produced and adopted by labs working on a wide range of projects within the field of vascular sciences and beyond.
We report that this device culture maintains arterial tissue viability for at least 7 days, and can be used to model specific steps of vascular disease. Using this, one could model physiological flow rates and pressure conditions13. Importantly, this perfusion culture is cost-effective due to the low production costs and the low volume of medium necessary to operate the system.
The 3D design can also be adapted to new applications, and new materials for printing can be tested. Even in its current format, the sample lodging space can be easily adapted to samples of different sizes by changing the length of the fittings or the bore of the luer connectors. It is important to note that, given the modular nature of the device and its small dimensions, this bioreactor can be used in several setups (Figure 5) and can be applied to multiplex cultures, where several samples can be exposed to different conditions at the same time in separate bioreactors.
It is envisioned that the use of the system be expanded in the future to support the culture of blood vessels from different origins (e.g., different species) and of different natures (e.g., veins, lymphatic), and perhaps applied to the culture of other hollow tissues (e.g., trachea, intestine). In particular, research shows that culturing tissue-engineered scaffolds in constant perfusion helps the homogenous distribution of cells within the construct and maturation of the resulting tissue25,26. In addition, seeding vascular grafts in perfusion contributes to achieving a more uniformly cellularized vascular lumen, as compared to static methods27. For this reason, we envision the system being applied to tissue engineering to help address the current challenges, allowing for the future development of reproducible synthetic blood vessel substitutes28.
The protocol described here presents some important steps critical to the success of the flow culture. Establishing and monitoring appropriate flow conditions is not trivial and needs to be performed on each system when it is set up for the first time, to ensure that the culture conditions are physiological. The flow and pressure were monitored using pressure sensors and ultrasound imaging. Another critical point is ensuring that the tissue is viable and intact at the beginning of the culture. This requires a fresh source, careful handling and can be verified by histological analysis. Additionally, troubleshooting must be performed at the start of every experiment to identify any potential bacterial contamination or media leakage source.
It is important to highlight that the perfusion system described, while providing physiological pressure and flow conditions, is unable to entirely mimic the complex pressure wave patterns recorded in vivo. This limitation is ascribable to using a peristaltic pump and can be resolved using more specialized equipment to reproduce advanced hemodynamic conditions. The culture of blood vessels in a bioreactor is also unable to address studies where the immune system or the interaction with other organs is critical.
In conclusion, a simple 3D printed perfusion system that can mimic the physiological hemodynamic environment is presented, which is expected to contribute to the standardization of ex vivo blood vessel cultures. Its potential for customization and application to long-term culture makes it an essential tool for advancing the understanding of these complex biological systems in physiology and pathological conditions.
Acknowledgements
The authors wish to thank the Veterinary Pathology Centre at the University of Surrey School of Veterinary Medicine for histology services. We also thank Drs L. Dixon, A. Reis, and M. Henstock from The Pirbright Institute (Pirbright, UK) for their support in procuring the animal tissues, and the Department of Biochemical Sciences at the University of Surrey, especially the technical team, for their continuing support. RSM was supported by the Doctoral College studentship award (University of Surrey), DM and PC were supported by the National Centre for the Replacement, Refinement & Reduction of Animals in Research (grant numbers: NC/R001006/1 and NC/T001216/1).
Materials
| Name | Company | Catalog Number | Comments |
| EasyFlow | - | - | 3D printed by MultiJet Fusion by Protolabs |
| PA12 - 3D printing | Protolabs | - | - |
| Peristaltic pump | Heidolph | PD5201 | |
| Culture media components: | |||
| Amphotericin B solution, 250 mug/mL in deionized water | Sigma-Aldrich | A2942-20ML | |
| Dextran from Leuconostoc spp. | Sigma-Aldrich | D8802-25ML | |
| Dulbecco's Modified Eagle's Medium - high glucose, w/ 4500 mg/L glucose, L-glutamine, sodium pyruvate, and sodium bicarbonate | Sigma-Aldrich | D6429-6X500ML | |
| Fetal Bovine Serum | Sigma-Aldrich | F9665 | |
| Penicillin-Streptomycin | Sigma-Aldrich | P4333-100ML | |
| Immunostaining materials: | |||
| Cryostat | LEICA | CM3050 S | |
| DAPI | Sigma-Aldrich | D9542-10MG | |
| Goat serum | Sigma-Aldrich | G9023-10ML | |
| Goat α-Rabbit Alexa Fluor 488 | Thermo Fisher Scientific | A11008 | |
| Invitrogen eBioscience Fluoromount G | Thermo Fisher Scientific | 50-187-88 | |
| MX35 Premier + Microtome Blade | Thermo Scientific | 3052835 | |
| Optimal Cooling Tempearure Compound - OCT | Agar Scientific | AGR1180 | |
| Rabbit α-CD31 antibody | Abcam | ab28364 | |
| Sudan Black B | Santa Cruz Biotechnology | SC-203760 | |
| X72 SuperFrost Plus Adhesion slide, 25x75x1mm, White, 90° Ground Edges, Frosted Area 20mm, 72/box | Fisher Scientific | J1800AMNZ | |
| α-Smooth Muscle Actin (SMA) Alexa Fluor® 647-conjugated antibody | R&D Systems | IC1420R | |
| Material for laser cutting of components: | |||
| Clear Plastic Sheet, 1250 mm x 610 mm x 1 mm (for laser cutting of washers) | RS Components | 258-6590 | |
| RS PRO Translucent Rubber Sponge Sheet, 600 mm x 600 mm x 1.5 mm (for laser cutting of silicone seals) | RS Components | 840-5541 | |
| Optional pressure monitors: | |||
| Pressure sensor | Parker Hannifin | 080-699PSX-3P-5 | |
| SciPres Pressure Monitor | Parker Hannifin | 206-200-M | |
| Pre-sterilized single use plasticware: | |||
| 0.2 um filter | Sarstedt | 70.1114.210 | |
| 20 mL Sterile syringe | IMS Euro | 40004 | |
| 50 mL Centrifuge Tube | Thermo Fisher Scientific | Sarstedt - 62.547.254 | |
| Small components: | |||
| Cable ties | - | - | |
| Masterflex Adapter Fittings, Female Luer to Hose Barb | Cole-Parmer | WZ-30800-10 | Barb Adaptor |
| Masterflex Polycarbonate Luer Fittings | Cole-Parmer | AU-45504-84 | |
| Nylon Miniature Check Valve | Cole-Parmer | 98553-00 | |
| RS PRO Translucent Rubber Sponge Sheet, 600 mm x 600 mm x 1.5 mm (for laser cutting of silicone seals) | RS Components | 840-5541 | |
| Stainless Steel M2 Hex Nuts | RS Components | 527-218 | |
| Stainless Steel M2 x 6 mm Screws | RS Components | 418-7426 | |
| Stainless Steel M5 Hex Nuts | RS Components | 189-585 | |
| Surgical vessel loop | Vascular Silicone Ties,International Medical Supplies | 10-1003 | |
| Three-way valves | IMS Euro | 91000 | |
| Surgical Equipment | |||
| Anatomical Forceps, GRAEFE, Curved, 10 cm SKU: BD-07 | International Medical Supplies | SKU: BD-07 | |
| Micro Forceps, Angled, 0.3 mm, 11 cm | International Medical Supplies | SKU: BD-361 | |
| Micro Scissors Noyes, Curved, 12 cm | International Medical Supplies | SKU: FD-12 | |
| Troge Surgical Scalpels - Size 23 - Box of 100 | International Medical Supplies | 63114 | |
| Tubing: | |||
| Eppendorf silicone tubing (I.D.1.6 mm, O.D.4.7 mm) | Eppendorf | M0740-2396 | System tubing |
| Masterflex PharMed BPT 3-Stop Tubing | ISMATEC | 95714-48 | Soft wall tubing (for clamp) |
| RS PRO Transparent Hose Pipe, 0.8 mm ID, Silicone | RS Components | 667-8432 | Resistance tubing (small inner diameter) |
| Tygon for food (I.D. 4.8 mm, W.T. 1.6 mm) | Heidolph | 525-30027-00-0 | One way valve tube |
| Verderflex Yellow Hose Pipe, 6.4 mm ID, Verderprene | RS Components | 125-4042 | Pump Tubing |
References
- Davies, P. F., Civelek, M., Fang, Y., Fleming, I. The atherosusceptible endothelium: Endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovascular Research. 99 (2), 315-327 (2013).
- Gugliandolo, E., et al. Palmitoylethanolamide and Polydatin combination reduces inflammation and oxidative stress in vascular injury. Pharmacological Research. 123, 83-92 (2017).
- Anselmino, M., et al. Catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction: A systematic review and meta-analysis. Circulation, Arrhythmia, and Electrophysiology. 7 (6), 1011-1018 (2014).
- Viola, M., et al. Subcutaneous delivery of monoclonal antibodies: How do we get there. Journal of Controlled Release. 286, 301-314 (2018).
- Kim, D. D. In vitro cellular models for nasal drug absorption studies. Drug Absorption Studies: In Situ, In Vitro and In Silico Models. , 216-234 (2008).
- Lewis, D. I. Animal experimentation: Implementation and application of the 3Rs. Emerging Topics in Life Sciences. 3 (6), 675-679 (2019).
- Rouwkema, J., et al. In vitro platforms for tissue engineering: Implications for basic research and clinical translation. Journal of Tissue Engineering and Regenerative Medicine. 5 (8), e164-167 (2011).
- Xu, Y., Shrestha, N., Préat, V., Beloqui, A. An overview of in vitro, ex vivo and in vivo models for studying the transport of drugs across intestinal barriers. Advanced Drug Delivery Reviews. 175, 113795 (2021).
- Vaghela, R., et al. Vessel grafts for tissue engineering revisited-Vessel segments show location-specific vascularization patterns in ex vivo ring assay. Microcirculation. 29 (2), e12742 (2022).
- Håkansson, J., et al. Individualized tissue-engineered veins as vascular grafts: A proof of concept study in pig. Journal of Tissue Engineering and Regenerative Medicine. 15 (10), 818-830 (2021).
- Saucy, F., et al. Ex vivo pulsatile perfusion of human saphenous veins induces intimal hyperplasia and increased levels of the plasminogen activator inhibitor 1. European Surgical Research. 45 (1), 50-59 (2010).
- Tosun, Z., McFetridge, P. S. Variation in cardiac pulse frequencies modulates vSMC phenotype switching during vascular remodeling. Cardiovascular Engineering and Technology. 6 (1), 59-70 (2015).
- Matos, R. S., Maselli, D., McVey, J. H., Heiss, C., Campagnolo, P. 3D printed bioreactor enabling the pulsatile culture of native and angioplastied large arteries. Frontiers in Cardiovascular Medicine. 9, 864580 (2022).
- Neff, L. P., et al. Vascular smooth muscle enhances functionality of tissue-engineered blood vessels in vivo. Journal of Vascular Surgery. 53 (2), 426-434 (2011).
- Boparai, K. S., Singh, R. Advances in Fused Deposition Modeling. In: Module. Refrence in Materials Science and Materials Engineering. , (2017).
- McKeen, L. W., McKeen, L. W. Chapter 6 - Polyamides (Nylons). The Effect of Creep and Other Time Related Factors on Plastics and Elastomers (Second Edition). , 197-262 (2012).
- Moradi, M., Mehrabi, O., Azdast, T., Benyounis, K. Y. Enhancement of low power CO2 laser cutting process for injection molded polycarbonate). Optics & Laser Technology. 96, 208-218 (2017).
- Ghasem, N. . Computer Methods in Chemical Engineering. , (2021).
- Lying, F., Gazi, F., Gardner, E. Preparation of tissues and cells for infrared and raman spectroscopy and imaging. Biomedical Applications of Synchrotron Infrared Microspectroscopy.RSC Analytical Spectroscopy Monographs. (11), 147-185 (2011).
- Sassi, L., et al. A perfusion bioreactor for longitudinal monitoring of bioengineered liver constructs. Nanomaterials. 11 (2), 275 (2021).
- Haykal, S., et al. Double-chamber rotating bioreactor for dynamic perfusion cell seeding of large-segment tracheal allografts: Comparison to conventional static methods. Tissue Engineering. Part C, Methods. 20 (8), 681-692 (2014).
- Kural, M. H., Dai, G., Niklason, L. E., Gui, L. An ex vivo vessel injury model to study remodeling. Cell Transplant. 27 (9), 1375-1389 (2018).
- Wong, M. M., Hong, X., Karamariti, E., Hu, Y., Xu, Q. Generation and grafting of tissue-engineered vessels in a mouse model. Journal of Visualized Experiments. (97), 52565 (2015).
- Alvino, V. V., et al. In vitro and in vivo preclinical testing of pericyte-engineered grafts for the correction of congenital heart defects. Journal of the American Heart Association. 9 (4), e014214 (2020).
- Nerurkar, N. L., Sen, S., Baker, B. M., Elliott, D. M., Mauck, R. L. Dynamic culture enhances stem cell infiltration and modulates extracellular matrix production on aligned electrospun nanofibrous scaffolds. Acta Biomaterialia. 7 (2), 485-491 (2011).
- Engebretson, B., Mussett, Z. R., Sikavitsas, V. I. The effects of varying frequency and duration of mechanical stimulation on a tissue-engineered tendon construct. Connective Tissue Research. 59 (2), 167-177 (2018).
- Saunders, S. K., et al. Evaluation of perfusion-driven cell seeding of small diameter engineered tissue vascular grafts with a custom-designed seed-and-culture bioreactor. PLoS One. 17 (6), e0269499 (2022).
- Stephenson, M., Grayson, W. Recent advances in bioreactors for cell-based therapies. F1000Research. 7, (2018).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved