A subscription to JoVE is required to view this content. Sign in or start your free trial.
Murine Hind Limb Explant Model for Studying the Mechanobiology of Achilles Tendon Impingement
In This Article
Summary
We present a custom experimental platform and tissue culture protocol that recreates fibrocartilaginous change driven by impingement of the Achilles tendon insertion in murine hind limb explants with sustained cell viability, providing a model suitable for exploring the mechanobiology of tendon impingement.
Abstract
Tendon impingement upon bone generates a multiaxial mechanical strain environment with markedly elevated transverse compressive strain, which elicits a localized fibrocartilage phenotype characterized by accumulation of glycosaminoglycan (GAG)-rich matrix and remodeling of the collagen network. While fibrocartilage is a normal feature in impinged regions of healthy tendons, excess GAG deposition and disorganization of the collagen network are hallmark features of tendinopathy. Accordingly, impingement is clinically recognized as an important extrinsic factor in the initiation and progression of tendinopathy. Nevertheless, the mechanobiology underlying tendon impingement remains understudied. Prior efforts to elucidate the cellular response to tendon impingement have applied uniaxial compression to cells and excised tendon explants in vitro. However, isolated cells lack a three-dimensional extracellular environment crucial to mechanoresponse, and both in vitro and excised explant studies fail to recapitulate the multiaxial strain environment generated by tendon impingement in vivo, which depends on anatomical features of the impinged region. Moreover, in vivo models of tendon impingement lack control over the mechanical strain environment. To overcome these limitations, we present a novel murine hind limb explant model suitable for studying the mechanobiology of Achilles tendon impingement. This model maintains the Achilles tendon in situ to preserve local anatomy and reproduces the multiaxial strain environment generated by impingement of the Achilles tendon insertion upon the calcaneus during passively applied ankle dorsiflexion while retaining cells within their native environment. We describe a tissue culture protocol integral to this model and present data establishing sustained explant viability over 7 days. The representative results demonstrate enhanced histological GAG staining and decreased collagen fiber alignment secondary to impingement, suggesting elevated fibrocartilage formation. This model can easily be adapted to investigate different mechanical loading regimens and allows for the manipulation of molecular pathways of interest to identify mechanisms mediating phenotypic change in the Achilles tendon in response to impingement.
Introduction
A multitude of tendons, including the Achilles tendon and rotator cuff tendons, experience bony impingement due to normal anatomical positioning1,2,3,4. Tendon impingement generates compressive strain directed transversely to the longitudinal fiber axis5,6,7. Regions of tendon impingement demonstrate a unique fibrocartilage phenotype in which shrunken, round cells (fibrochondrocytes) are embedded within a disorganized collagen network with markedly increased glycosaminoglycan (GAG) content2,3,4,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24. Prior studies suggest the disparate mechanical environment produced by tendon impingement sustains this GAG-rich matrix by driving the deposition of large aggregating proteoglycans, most notably aggrecan, although the underlying mechanisms are unclear1,3,12,13,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39. While fibrocartilage is a normal feature in impinged regions of healthy tendons, aberrant proteoglycan metabolism associated with excessive fibrocartilage formation is a hallmark feature of tendinopathy, a common and debilitating disease that disproportionately emerges in chronically impinged tendons1,40,41,42,43,44,45,46,47,48,49. Accordingly, tendon impingement is clinically recognized as an important extrinsic factor driving several of the most common tendinopathies, including rotator cuff disease and insertional Achilles tendinopathy (IAT)50,51,52. Currently, treatment of tendinopathy is inefficient. For example, approximately 47% of patients with IAT require surgical intervention after failed conservative management, with variable postoperative outcomes53,54,55,56. Despite the apparent relationship between impingement and tendinopathy, the mechanobiological mechanisms by which cells in impinged tendon sense and respond to their mechanical environment are poorly described, which obscures understanding of tendinopathy pathogenesis and results in inadequate treatment.
Explant models are useful tools in the study of tendon mechanobiology57,58. As a first step towards understanding the mechanobiology of tendon impingement, several prior studies have explored cellular response following the application of simple uniaxial compression to cells or excised tendon explants27,29,30,31,32,33,34,39. However, cells in vitro lack extracellular and pericellular matrices that facilitate strain transfer, sequester important growth factors and cytokines released by mechanical deformation, and provide substrate for focal adhesion complexes that play a role in mechanotransduction57,59. Additionally, both in vitro and excised explant studies fail to recapitulate the multiaxial mechanical strain environment generated by tendon impingement in vivo, which depends on anatomical features of the impinged region5,6. In the context of the impinged Achilles tendon insertion, this includes surrounding tissues such as the retrocalcaneal bursa and Kager's fat pad60,61,62,63. Conversely, in vivo models of tendon impingement25,28,36,37,38,64,65,66 allow minimal control over the magnitude and frequency of load applied directly to the tendon, which is a well-recognized limitation of in vivo models for studying tendon mechanobiology57,58,67,68,69,70. Given challenges in measuring tendon strain in vivo, the internal strain environment generated within these models is often poorly characterized.
In this manuscript, we present a custom experimental platform that recreates impingement of the Achilles tendon insertion upon the calcaneus within whole murine hind limb explants that, when paired with this tissue culture protocol, maintains viability over 7 days in explant culture and allows for study of the biologic sequelae of tendon impingement. The platform is built upon a 3D printed polylactic acid (PLA) base that provides the foundation for the attachment of the grips and 3D printed PLA volume reduction insert. The grips are used to clamp the upper leg and knee proximal to the Achilles myotendinous junction with the caudal aspect of the hind limb facing upward, allowing the Achilles tendon to be imaged from above using an ultrasound probe or inverted microscope (Figure 1A). The volume reduction insert slides along a track on the base and reduces the required volume of tissue culture media. A braided line wrapped around the hind paw is routed out of the platform utilizing the base design and a 3D printed PLA clip. By pulling on the string, the hind paw is dorsiflexed, and the Achilles tendon insertion is impinged against the calcaneus, resulting in elevated transverse compressive strain5,6 (Figure 1A). The platform is contained within an acrylic bath that maintains the hind limb explants submerged in tissue culture media. Securing the taut string to the outside of the bath with adhesive tape maintains ankle dorsiflexion to produce static impingement of the Achilles tendon insertion. CAD files for 3D printed components are provided in multiple formats (Supplementary File 1), allowing import into a range of commercial and free, open-source CAD software for modification to suit experimental needs. If access to 3D printers is not available for fabrication, CAD files can be provided to online 3D printing services that will print and ship the parts at low cost.
Importantly, the triceps surae-Achilles musculotendinous complex spans both the knee and ankle joints71,72,73. Consequently, tensile strain in the Achilles tendon is influenced by knee flexion. Knee extension places the Achilles tendon under tension, whereas knee flexion reduces tension. By first extending the knee and then passively dorsiflexing the ankle, compressive strains at the impinged insertion can be superimposed upon tensile strains. Conversely, by passively dorsiflexing the ankle with the knee flexed, tensile strain is reduced, and compressive strain remains. The current protocol explores three such conditions. 1) For static impingement, the foot is dorsiflexed to < 110° with respect to the tibia to impinge the insertion, with the knee flexed to reduce tension. 2) For the baseline tension group, the ankle is extended above 145° of dorsiflexion with the knee extended, generating predominately tensile strain at the insertion. 3) For the unloaded group, explants are cultured in a Petri dish with the knee and ankle in neutral positions in the absence of externally applied load. The angles referred to above are photographically measured relative to a coordinate system where the foot and tibia are parallel at an angle of 180° and perpendicular at an angle of 90°.
Key steps of the protocol include 1) dissection of hind limb explants and careful removal of the skin and plantaris tendon; 2) explant culture following a 48 h dexamethasone pretreatment; 3) tissue sectioning and histological staining; and 4) color image analysis to assess fibrocartilage formation. Following dissection, each hind limb explant is pretreated for 48 h in culture media supplemented with dexamethasone74. Contralateral limbs from each mouse are assigned to separate experimental groups for pairwise comparison, which helps control biological variability. After pretreatment, explants are positioned into platforms as described above and cultured for 7 more days (Figure 1B). Additional comparisons are made to a pretreated (day 0) group in which explants are removed immediately following the 48 h pretreatment.
After explant culture, hind limbs are trimmed down, formalin fixed, decalcified and embedded in paraffin. Serial sectioning in sagittal orientation provides visualization of the Achilles tendon from the myotendinous junction to the calcaneal insertion while allowing section depth to be tracked through the entire tendon. Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP X-nick labeling (TUNEL) is used to visualize DNA damage secondary to apoptosis and assess viability. Toluidine blue histology and custom color image analysis are performed to quantify changes in GAG staining. Toluidine blue stained tissue sections are then used for SHG imaging to characterize alterations in collage fiber organization (Figure 1B).
The provided representative results suggest altered histological staining of the GAG-rich matrix and disorganization of the extracellular collagen network generated by 7 days of static impingement within the model. This model can be utilized to explore molecular mechanisms underlying impingement-driven fibrocartilaginous change.
Protocol
All animal work was approved by the University of Rochester Committee on Animal Resources.
1. Preparation of tissue culture media
- Culture all explants in Dulbecco's Modified Eagle Medium (1x DMEM) with 1% v/v penicillin-streptomycin and 200 µM L-ascorbic acid in an incubator at 37 °C and 5% CO2. For the initial 48 h pretreatment, culture each explant in 70 mL of culture media supplemented with 100 nM dexamethasone74. After pretreatment, culture limbs for 7 more days without dexamethasone, changing media every 48-72 h.
NOTE: Addition of serum, such as fetal bovine serum, to the culture media is not advised consistent with recommendations provided by Wunderli, Blache, and Snedeker57. In brief, serum-free conditions better represent the avascular, nutrient-poor tendon microenvironment that exists in vivo. Furthermore, serum supplementation can promote tissue degradation under certain culture conditions75 and stimulate cell proliferation and migration out of tissue, both features of tendon pathology57. - For the unloaded group, culture each explant in 70 mL of media. For the baseline tension and static impingement groups, each platform requires approximately 125 mL of culture media to keep the limb submerged. This volume can vary depending on the positioning of the upper leg in the grips and 3D print parameters, primarily the infill density.
2. Explant dissection and dexamethasone pretreatment
- Euthanize mice via CO2 inhalation and secondary cervical dislocation, or according to institutional guidelines. This protocol utilizes C57BL/6 mice less than 1 year old. Hind limb size will increase with age and may become challenging to fit within the grips.
- Prior to dissection, transfer 70 mL of prewarmed (37 °C) culture media into a 100 mm (diameter) x 25 mm (height) Petri dish in a sterile biological safety cabinet (BSC). Add dexamethasone to achieve 100 nM working concentration.
- Dissections can be carried out on the benchtop using absorbent underpads, working swiftly through the dissection before transferring hind limb explants into the BSC. Assemble the surgical tools required for this dissection which include smooth, straight, fine tip forceps; straight, fine tip forceps with serrated teeth; and straight, sharp, fine scissors.
- For dissection of hind limb explants, place the mouse in a supine position and identify the hip joint. Using fine scissors, make a small (5-10 mm) incision through the skin overlaying the proximal and anterior (cranial) aspect of the upper leg.
- Pull the incision apart to expand, pinch the exposed upper leg using fingers, and carefully pull the skin distally to deglove the hind limb to the level of the ankle. Gently insert one scissor blade under the skin along the dorsal aspect of the foot and make an incision extending to the toes. Continue to pull the skin distally to completely remove.
- Position the mouse to visualize the Achilles tendon insertion onto the posterior (caudal) aspect of the calcaneus, near the ankle. Proximal to the Achilles tendon insertion, the plantaris tendon lies directly adjacent to the medial border of the Achilles tendon and extends distally toward the plantar aspect of the foot, passing over the posterior aspect of the calcaneus.
- To remove the plantaris tendon, carefully insert one tip of the smooth, fine tip forceps between the two tendons and extend the tip medially passing underneath the plantaris tendon. Draw the tip proximally and tear through the plantaris muscle. Using fine tip, serrated forceps, grab the detached proximal end of the plantaris tendon and pull distally to remove.
- At the hip joint, use fine scissors to cut through the pelvis and isolate the hind limb. Use the scissors to pry off the remaining pelvis and expose the femoral head.
- Transfer the hind limb explant to the BSC and into the dish containing cell culture media with dexamethasone. Move the dish into the incubator and pretreat for 48 h.
3. Explant culture and loading platforms
- As the 48 h pretreatment concludes, prewarm sufficient volumes of culture media (section 1). From this point forward, no dexamethasone will be added to culture media. At this time, limbs from the pretreated (day 0) group can be fixed, decalcified, and embedded in paraffin for future sectioning, staining and analysis.
- For the unloaded group, aspirate pretreatment media and transfer explants to fresh Petri dishes, add 70 mL of culture media each, and return to the incubator.
- For the baseline tension and static impingement groups, prepare explant platforms. Cut pieces of sandpaper similar in size to the grip platens. For the static impingement group, cut pieces of braided line approximately 18 inch in length and pre-tie a loose overhand knot halfway along the length of line. Tear pieces of aluminum foil to cover each acrylic bath and spray with 70% ethanol (EtOH). Transfer preparations to the BSC.
- Each platform includes an acrylic bath, base, volume reduction insert, clip, and grips. Each grip includes two platens and three different types of screws including one M5 x 0.8 mm thread x 10 mm long screw that attaches the grips to the base; two M6 x 1 mm thread x 20 mm long screws that extend the platens to clamp the grips; and four M3 x 0.5 mm thread x 14 mm long screws that, in combination with four compression springs, retract the platens to open the grips.
- Place all components into secondary containers capable of capturing all culture media in the event of a leak. Submerge in ≥ 10% bleach solution and soak for at least 1 h. Rinse off bleach solution with tap water (autoclave as needed) and move into the BSC.
NOTE: For troubleshooting contamination, consider autoclaving all tap water or using purified water. See the Discussion for additional tips when addressing contamination. - Use the M3 screws and compression springs to attach platens to the grips, secure the grips to the base using the M5 screw, and insert the M6 screws until they engage the platens. Use double-sided tape to attach sandpaper to the platens, then close the grips to promote adhesion of the sandpaper to the platens. Repeat for all platforms.
- When ready to load a platform, fully open the grips and using forceps, place the upper leg and knee between the platens with the superficial surface of the Achilles tendon facing upward (Figure 1A). Loosely close the grips to gently hold in place.
- Use forceps to grab the exposed femoral head or foot and manipulate the knee flexion angle as you gradually close the grips to secure in place. For the baseline tension group, extend the knee joint as described previously. As the grips tighten with the knee extended, the ankle should naturally extend. For the static impingement group, flex the knee joint between the grips as described previously.
- For the static impingement group, place the overhand knot of the string around the distal paw and tighten. Route the string through a slot in the base located underneath the explant and through the clip hole. Place the base into the acrylic bath and secure the clip to the top edge of the bath (Figure 1A).
- Pull the string to dorsiflex the foot to at least 110° with respect to the tibia and use a permanent marker to mark the string as it exits the clip. Take a photograph of the explant in this position to later quantify dorsiflexion angle (Figure 1A).
- Remove the base and attach the volume reduction insert by sliding along a track on the base. Place the base (now attached to the volume reduction insert) back into the acrylic bath and reposition the clip on the top edge. Pull the string to return to the original dorsiflexion angle using the marked string as a guide and secure the string to the outside of the bath with tape to maintain static dorsiflexion.
- For the baseline tension group, simply place the base with volume reduction insert into the acrylic bath once the explant is positioned between the grips and take a photograph for quantification of dorsiflexion angle.
- Add 125 mL of prewarmed (37 °C) culture media to each platform to submerge explants. Cover the top of the bath with aluminum foil, place in a secondary container, and move into the incubator. Culture for 7 additional days, changing media every 48-72 h.
NOTE: Tape placed across the top of the bath can prevent PLA parts from floating.
4. Fixation, decalcification and paraffin embedding
- Following explant culture, use scissors to trim toenails/distal toes and cut away the upper leg proximal to the Achilles myotendinous junction. Place each trimmed ankle joint into a processing cassette lined with foam biopsy pads. Push the ankle into the cassette corner to position the ankle at approximately 90° dorsiflexion and close the cassette to keep in place.
- Fix for 3 days in 10% neutral buffered formalin (NBF) and decalcify for 2 weeks in 14% ethylenediamenetetraacetic acid (EDTA) dissolved in distilled water (diH2O) with pH adjusted to 7.4-7.6 with glacial acetic acid.
- To remove salts, thoroughly rinse samples three times in both 1x phosphate buffered saline (PBS) followed by diH2O, 5 minutes each. Perform routine sample processing for paraffin histology: dehydrate through a graded series of EtOH, clear in xylene, and infiltrate with paraffin wax. Orient and embed samples in paraffin to obtain sagittal tissue sections through the Achilles tendon in medial-to-lateral progression as described in section 5 below (Figure 1B).
5. Tissue sectioning
- With a microtome, carefully trim into the sample until sections are parallel to the block face. Grossly trim into the ankle from the medial aspect of the joint, stopping before reaching the medial border of the Achilles tendon insertion.
- Transfer the sample to an ice block to adjust temperature and hydration, and change blades (or shift to a fresh section of the current blade). Continue sectioning into the sample at 10 µm thickness and carefully identify entry into the Achilles tendon insertion with a brightfield microscope. Once identified, perform serial sectioning tracking section number (i.e., tissue depth) through the entire Achilles tendon insertion.
6. Deparaffinization/rehydration and slide selection
- For each assay below, select level-matched tissue sections from each pair of contralateral limbs. Prior to staining, place on a slide rack and move through 3 changes of xylene, 2 changes of 100% EtOH, 2 changes of 95% EtOH, and 1 change of 70% EtOH, 5 min each. Finish rehydrating in diH2O.
7. TUNEL to assess Achilles tendon viability
- For TUNEL labeling, stain according to the manufacturer's protocol. Incubate in 20 µg/mL proteinase K for 20 min at room temperature and rinse in diH2O. Incubate in 50 µL of TUNEL stain solution (5 µL enzyme solution, 45 µL label solution) for 1 h at 37 °C. Rinse in diH2O. Mount with antifade reagent containing DAPI and coverslip.
- Image the Achilles tendon insertion using a fluorescence microscope with a 4x objective lens. Include a DAPI channel (excitation/emission wavelengths = 360/460 nm) to visualize all nuclei, a TUNEL (TMR Red) channel (excitation/emission wavelengths = 540/580 nm) to visualize apoptotic nuclei, and if possible, a brightfield channel.
- For image analysis, import images into an image analysis software suitable for ROI-based processing, such as FIJI/ImageJ or MATLAB. Define a region of interest (ROI) outlining the entire Achilles tendon in view (Figure 2A), excluding cells in the epitenon that are highly susceptible to death induced by dissection and sudden change in environmental conditions when placed into culture.
- To do this, perform ROI selection in MATLAB by importing the brightfield image and using the drawpolygon() function to trace and enclose the boundaries of the tendon. MATLAB creates a Polygon object for the ROI, which can then be applied to the DAPI and TUNEL channel images to mask out pixel intensity data outside the ROI using createMask() in order to only analyze nuclei within the Achilles tendon.
- Import the DAPI and TUNEL channel images and identify a fluorescence intensity threshold for defining apoptotic (TUNEL+) nuclei by normalizing to the maximum fluorescence intensity of apoptotic nuclei in nonviable tissues such as bone, muscle or fat that are incidentally captured in the tissue section. Once the images are masked, calculate the fraction of apoptotic nuclei (TUNEL+ nuclei/DAPI nuclei) within the Achilles tendon.
8. Toluidine blue histology to characterize fibrocartilage formation
- Once rehydrated (Section 6), transfer slide rack with level-matched tissue sections from contralateral pairs of explants to 0.4% w/v Toluidine blue O in 0.1 M sodium acetate buffer with pH adjusted to 4.0 using glacial acetic acid. Incubate for 10 min at room temperature, then rinse 3x in diH2O for 30 s each.
- Dehydrate through three changes of 95% EtOH and two changes of 100% EtOH, 30 s each. Clear through three changes of xylene, 1 min each. Coverslip with xylene-based mounting medium.
- Obtain 24-bit Red-Blue-Green (RGB) color images of the Achilles tendon insertion. For example, for this protocol use an adapter to interface a digital color camera to the eyepiece of a simple brightfield microscope with a 4x objective.
- To quantify differences in Toluidine blue staining within the compressive tendon fibrocartilage (CTF)16 at the Achilles tendon insertion (Figure 3A,B), import RGB images into an image analysis software capable of defining and managing multiple ROIs. Options include the selections and ROI manager tools in FIJI/ImageJ or the image processing toolbox in MATLAB. Begin by setting the pixel/length scale.
NOTE: Software choice is left to the discretion of the researcher and is certainly not limited to MATLAB or FIJI/ImageJ. The authors have provided MATLAB code (Supplementary File 2), documentation describing implementation (Supplementary File 3), and a sample image (Supplementary File 4). We encourage researchers to translate this code to alternative programming languages for use in other software as needed or preferred. - With the image displayed in the software of choice, identify the intersection of the deep tendon border with the calcaneus. From here, trace proximally 800 µm along the deep tendon border to establish the deep boundary of the CTF. For example, use the drawpolyline() function in MATLAB to interactively draw a polyline over the RGB image. MATLAB creates a Polyline object containing the vertices of the line, which can be processed and trimmed to a length of 800 µm.
- From this position, create the proximal boundary of the CTF by drawing a line segment connecting to the superficial tendon border that is perpendicular to the local fiber orientation.
- Move back to the intersection of the deep tendon border and the calcaneus and define the distal boundary of the CTF by drawing a line segment connecting to the superficial tendon border along the distinct tidemark separating the CTF from the attachment zone fibrocartilage (AZF)16 (Figure 3A,B). Lastly, generate the superficial boundary of the CTF by tracing the superficial tendon border to enclose.
- To describe spatial variations in GAG staining across the insertion, divide the total CTF into 4 quadrants (Figure 3A,B). Connect the midpoint of the deep and superficial CTF boundaries with a line segment to create a distal/proximal boundary. Passing through the midpoint of this boundary, connect the midpoints of the distal and proximal CTF boundaries along the fiber orientation to create a superficial/deep boundary.
- These 6 boundaries provide information that can be used to define the ROI representing the entire CTF, but also 4 quadrants subdividing the CTF. In MATLAB, for example, compile vertices defining the boundaries of each individual ROI (CTF, quadrants 1-4) into vectors and use images.roi.Polygon() to generate enclosed Polygon objects for each ROI.
- With ROIs defined, transform RGB pixel data into the Hue-Saturation-Value (HSV) color space using the color transformer plugin in FIJI, the rgb2hsv() function in MATLAB, or other software applying the appropriate transformation equations76. HSV data can then be projected into the 2D hue-saturation space, where each combination of hue and saturation encode a unique color (Figure 3C).
- Within each ROI, calculate the average hue and saturation that describe the average stain color in the ROI. This can be achieved in MATLAB, for example, by using the Polygon objects defining each ROI to mask the image using createMask() in order to analyze hue-saturation pixel data specifically within each ROI.
- Define another small ROI within the periosteal fibrocartilage (PF)16 (Figure 3A, B) and calculate average hue and saturation. Then, calculate the Euclidean distance separating the average color in each ROI of the CTF to that of the PF (Figure 3C).
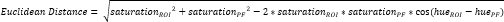
NOTE: The Euclidean distance calculation describes the degree of similarity between the average color of the ROI to that of the PF, a quintessential fibrocartilaginous tissue16,17,77. A smaller Euclidean distance indicates a ROI color that is more fibrocartilaginous in appearance. - Use this distance to calculate fibrocartilage score, which assumes a maximum value of 1 if the ROI color is identical to the PF color and a minimum value of -1 if the ROI and PF color reach maximum separation within the hue-saturation color space.
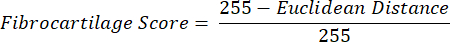
- Average data for hue, saturation, and fibrocartilage score within each ROI across tissue sections from each limb. Perform paired statistical comparisons between groups of contralateral limbs.
9. SHG imaging to investigate change in collagen network organization
- Perform SHG imaging of the Toluidine blue stained sections using a capable microscope system with a 20x object lens. Acquire z-stacks through the section thickness and perform tile scanning as needed to fully capture the Achilles tendon insertion.
- Import SHG images into a preferred image analysis software and define ROIs encompassing and subdividing the CTF at the Achilles tendon insertion as described for the Toluidine blue image analysis in section 8 (Figure 4A,B).
- If SHG images are imported into MATLAB, use the approach for defining CTF ROIs in MATLAB as outlined in section 8 . After defining ROIs, transfer ROI coordinates into FIJI by exporting ROI vertices from MATLAB as .txt files and importing into FIJI using File > Import > XY Coordinates. Overlay the selection and send to the ROI manager for analysis.
- To quantify collagen organization, use the Directionality plugin in FIJI, which performs Fourier spectrum analysis to calculate distributions of fiber orientations across small windows spanning a ROI. The spread of this distribution, referred to as dispersion, is inversely related to fiber alignment.
NOTE: Collagen fibers may assume variable orientations in different windows across the CTF due to gross curvature of the tendon rather than the absence of alignment/organization. To better distinguish changes in collagen organization from gross curvature of the tendon at the insertion, it is necessary to define smaller ROIs. - Import SHG images into FIJI and set the pixel/length scale. Project maximum pixel intensity data into a 2D composite image and add ROIs to the ROI Manager. Sequentially overlay each CTF ROI onto the image and draw 10 small sub-ROIs of consistent size within the ROI, adding the sub-ROIs to the ROI manager.
- Within each sub-ROI, run the directionality plugin in FIJI to calculate fiber dispersion. Average dispersion data across sub-ROIs within each CTF ROI, and average dispersion data within each CTF ROI across sections from each explant. Perform paired statistical comparisons between groups of contralateral limbs.
Results
Representative images of TUNEL stained tissue sections demonstrate minimal apoptotic nuclei within the body of the Achilles tendon after 7 days of explant culture across experimental groups (Figure 2A). Quantification of these images provides evidence that the tissue culture protocol maintains up to 78% viability on average within the Achilles tendon after 7 days of explant culture across loading conditions (Figure 2B).
Qualitatively,...
Discussion
The experimental murine hind limb explant platform paired with the tissue culture protocol described in this study provide a suitable model for studying the mechanobiology of impingement-driven fibrocartilage formation at the Achilles tendon insertion. The utility of this explant model is demonstrated by the representative results, which indicate maintenance of cell viability concomitant with significant and spatially heterogeneous change in Toluidine blue staining after 7 days of static impingement. These findings sugge...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors are grateful for support and assistance provided by Jeff Fox and Vidya Venkatramani of the University of Rochester Center for Musculoskeletal Research's Histology, Biochemistry, and Molecular Imaging (HBMI) Core, funded in part by P30AR06965. Additionally, the authors would like to thank the Center for Light Microscopy and Nanoscopy (CALMN) at the University of Rochester Medical Center for assistance with multiphoton microscopy. This study was funded by R01 AR070765 and R01 AR070765-04S1, as well as 1R35GM147054 and 1R01AR082349.
Materials
| Name | Company | Catalog Number | Comments |
| Absorbent underpads | VWR | 82020-845 | For benchtop dissection |
| Acrylic bath | Source One | X001G46CB1 | Contains the explant platform submerged in culture media |
| Autoclave bin | Thermo Scientific | 13-361-20 | Used as secondary containment, holds two platforms |
| Base | - | - | 3D printed from CAD files provided as Supplementary Files |
| Braided line | KastKing | 30lb test | Used to wrap around paw and apply ankle dorsiflexion |
| Clip | - | - | 3D printed from CAD files provided as Supplementary Files |
| Cover glass | Fisherbrand | 12-541-034 | Rectangular, No. 2, 50 mm x 24 mm |
| Cytoseal XYL | VWR | 8312-4 | Xylene-based mounting media for coverslipping Toluidine blue stained tissue sections |
| Dexamethasone | MP Biomedical LLC | 194561 | CAS#50-02-2 |
| Dimethyl sulfoxide (DMSO), anhydrous | Invitrogen by ThermoFisher | D12345 | CAS#67-68-5, use to solubilize dexamethasone into concentrated stock solutions |
| Double-sided tape | Scotch Brand | 34-8724-5195-9 | To attach sandpaper to Grip platens |
| Dulbecco's Modified Eagle Medium (1X DMEM) | Gibco by ThermoFisher | 11965092 | high glucose, (-) pyruvate, (+) glutamine |
| EDTA tetrasodium salt dihydrate | Thermo Scientific Chemicals | J15700.A1 | CAS#10378-23-1, used to make 14% EDTA solution for sample decalcifcation |
| Ethanol, 200 proof | Thermo Scientific | T038181000 | CAS#64-17-5, 1 L supply |
| Foam biopsy pads | Leica | 3801000 | Used with processing cassettes, help hold ankle joints in desired position during fixation and decalcification |
| Forceps, #SS Standard Inox | Dumont | 11203-23 | Straight, smooth, fine tips |
| Forceps, Micro-Adson 4.75" | Fisherbrand | 13-820-073 | Straight, fine tips with serrated teeth |
| Garnet Sandpaper, 50-D Grit | Norton | M600060 01518 | Or other coarse grit sandpaper |
| Glacial acetic acid | Fisher Chemical | A38S-500 | CAS#64-19-7, for adjusting pH of sodium acetate buffer used for Toluidine blue histology, as well as 14% EDTA decalcification solution |
| Grips | ADMET | GV-100NT-A4 | Stainless steel vice grips, screws and springs described in the protocol are included |
| Histobond Adhesive Microscope Slides | VWR | 16005-108 | Sagittal sections of hind limbs explants reliably adhere to these slides through all staining protocols |
| In situ Cell Death Detection Kit, TMR Red | Roche | 12156792910 | TUNEL assay |
| Labeling tape | Fisherbrand | 15-959 | Or any other labeling tape of preference |
| L-ascorbic acid | Sigma-Aldrich | A4544-100G | CAS#50-81-7, for culture media formulation |
| Neutral buffered formalin, 10% | Leica | 3800600 | For sample fixation, 5 gallon supply |
| Nunc petri dishes | Sigma-Aldrich | P7741-1CS | 100 mm diameter x 25 mm height, maintain explants submerged in 70 mL of culture media as described in protocol |
| Penicillin-streptomycin (100X) | Gibco by ThermoFisher | 15140122 | Add 5 mL to 500 mL 1X DMEM for 1% v/v (1X) working concentration |
| Polylactic acid (PLA) 1.75 mm filament | Hatchbox | - | Choose filament diameter compatible with your 3D printer extruder, in color of choice. |
| Processing cassettes | Leica | 3802631 | For fixation, decalcification and paraffin embedding |
| Prolong Gold Antifade Reagent with DAPI | Invitrogen by ThermoFisher | P36931 | Mounting media for coverslipping tissue sections after TUNEL |
| Proteinase K | Fisher BioReagents | BP1700-50 | CAS#39450-01-6, used for antigen retrieval in TUNEL protocol |
| Scissors, Fine | FST | 14094-11 | Straight, sharp |
| Slide Staining Set, 12-place | Mercedes Scientific | MER 1011 | Rack with 12 stain dishes and slide dippers for Toluidine blue histology |
| Sodium acetate, anhydrous | Thermo Scientific Chemicals | A1318430 | CAS#127-09-3, used to make buffer for Toluidine blue histology |
| Tissue-Tek Accu-Edge Low Profile Microtome Blades | VWR | 25608-964 | For paraffin sectioning |
| Toluidine Blue O | Thermo Scientific Chemicals | 348601000 | CAS#92-31-9 |
| Volume Reduction Insert | - | - | 3D printed from CAD files provided as Supplementary Files |
| Xylenes | Leica | 3803665 | 4 gallon supply for histological staining |
References
- Cook, J. L., Purdam, C. Is compressive load a factor in the development of tendinopathy. Br J Sports Med. 46 (3), 163-168 (2012).
- Benjamin, M., Qin, S., Ralphs, J. R. Fibrocartilage associated with human tendons and their pulleys. J Anat. 187 (Pt 3), 625-633 (1995).
- Benjamin, M., Ralphs, J. R. Fibrocartilage in tendons and ligaments - an adaptation to compressive load. J Anat. 193 (4), 481-494 (1998).
- Benjamin, M., Theobald, P., Suzuki, D., Toumi, H. The anatomy of the Achilles tendon. The Achilles Tendon. 3, 5-16 (2007).
- Chimenti, R. L., et al. Insertional achilles tendinopathy associated with altered transverse compressive and axial tensile strain during ankle dorsiflexion. J Orthop Res. 35 (4), 910-915 (2017).
- Mora, K. E., et al. Ultrasound strain mapping of the mouse Achilles tendon during passive dorsiflexion. J Biomech. 132, 110920 (2022).
- Pringels, L., et al. Intratendinous pressure changes in the Achilles tendon during stretching and eccentric loading: Implications for Achilles tendinopathy. Scand J Med Sci Sports. 33 (5), 619-630 (2023).
- Koob, T. J., Vogel, K. G. Site-related variations in glycosaminoglycan content and swelling properties of bovine flexor tendon. J Orthop Res. 5 (3), 414-424 (1987).
- Vogel, K. G., Koob, T. J. Structural specialization in tendons under compression. Int Rev Cytol. 115, 267-293 (1989).
- Vogel, K. G., Ordög, A., Pogány, G., Oláh, J. Proteoglycans in the compressed region of human tibialis posterior tendon and in ligaments. J Orthop Res. 11 (1), 68-77 (1993).
- Vogel, K. G., Sandy, J. D., Pogány, G., Robbins, J. R. Aggrecan in bovine tendon. Matrix Biol. 14 (2), 171-179 (1994).
- Robbins, J. R., Vogel, K. G. Regional expression of mRNA for proteoglycans and collagen in tendon. Eur J Cell Biol. 64 (2), 264-270 (1994).
- Vogel, K., Gordon, S. I., Blair, S. J., Fine, L. J. . Repetitive motion disorders of the upper extremity. , (1995).
- Benjamin, M., Tyers, R. N., Ralphs, J. R. Age-related changes in tendon fibrocartilage. J Anat. 179, 127-136 (1991).
- Ralphs, J. R., Benjamin, M., Thornett, A. Cell and matrix biology of the suprapatella in the rat: a structural and immunocytochemical study of fibrocartilage in a tendon subject to compression. Anat Rec. 231 (2), 167-177 (1991).
- Rufai, A., Benjamin, M., Ralphs, J. R. Development and ageing of phenotypically distinct fibrocartilages associated with the rat Achilles tendon. Anat Embryol (Berl). 186 (6), 611-618 (1992).
- Rufai, A., Ralphs, J. R., Benjamin, M. Ultrastructure of fibrocartilages at the insertion of the rat Achilles tendon. J Anat. 189 (Pt 1), 185-191 (1996).
- Waggett, A. D., Ralphs, J. R., Kwan, A. P. L., Woodnutt, D., Benjamin, M. Characterization of collagens and proteoglycans at the insertion of the human achilles tendon. Matrix Biol. 16 (8), 457-470 (1998).
- Ralphs, J., et al. Regional differences in cell shape and gap junction expression in rat Achilles tendon: relation to fibrocartilage differentiation. J Anat. 193 (pt 2), 215-222 (1998).
- Milz, S., et al. Three-dimensional reconstructions of the Achilles tendon insertion in man. J Anat. 200 (Pt 2), 145-152 (2002).
- Tischer, T., Milz, S., Maier, M., Schieker, M., Benjamin, M. An immunohistochemical study of the rabbit suprapatella, a sesamoid fibrocartilage in the quadriceps tendon containing aggrecan. J Histochem Cytochem. 50 (7), 955-960 (2002).
- Esquisatto, M. A., Joazeiro, P. P., Pimentel, E. R., Gomes, L. The effect of age on the structure and composition of rat tendon fibrocartilage. Cell Biol Int. 31 (6), 570-577 (2007).
- Matuszewski, P. E., et al. Regional variation in human supraspinatus tendon proteoglycans: Decorin, biglycan, and aggrecan. Connect Tissue Res. 53 (5), 343-348 (2012).
- Buckley, M. R., Huffman, G. R., Iozzo, R. V., Birk, D. E., Soslowsky, L. J. The location-specific role of proteoglycans in the flexor carpi ulnaris tendon. Connect Tissue Res. 54 (6), 367-373 (2013).
- Gillard, G. C., Reilly, H. C., Bell-Booth, P. G., Flint, M. H. The influence of mechanical forces on the glycosaminoglycan content of the rabbit flexor digitorum profundus tendon. Connect Tissue Res. 7 (1), 37-46 (1979).
- Giori, N. J., Beaupre, G. S., Carter, D. R. Cellular shape and pressure may mediate mechanical control of tissue composition in tendons. J Orthop Res. 11 (4), 581-591 (1993).
- Wren, T. A., Beaupré, G. S., Carter, D. R. Mechanobiology of tendon adaptation to compressive loading through fibrocartilaginous metaplasia. J Rehabil Res Dev. 37 (2), 135-143 (2000).
- Malaviya, P., et al. An in vivo model for load-modulated remodeling in the rabbit flexor tendon. J Orthop Res. 18 (1), 116-125 (2000).
- Shim, J. W., Elder, S. H. Influence of Cyclic Hydrostatic Pressure on Fibrocartilaginous Metaplasia of Achilles Tendon Fibroblasts. Biomech Model Mechanobiol. 5 (4), 247-252 (2006).
- Koob, T. J., Clark, P. E., Hernandez, D. J., Thurmond, F. A., Vogel, K. G. Compression loading in vitro regulates proteoglycan synthesis by tendon fibrocartilage. Arch Biochem Biophys. 298 (1), 303-312 (1992).
- Evanko, S. P., Vogel, K. G. Proteoglycan Synthesis in Fetal Tendon Is Differentially Regulated by Cyclic Compression in Vitro. Arch Biochem Biophys. 307 (1), 153-164 (1993).
- Vogel, K. G. The effect of compressive loading on proteoglycan turnover in cultured fetal tendon. Connect Tissue Res. 34 (3), 227-237 (1996).
- Thornton, G. M., et al. Changes in mechanical loading lead to tendon specific alterations in MMP and TIMP expression: influence of stress deprivation and intermittent cyclic hydrostatic compression on rat supraspinatus and Achilles tendons. Br J Sports Med. 44 (10), 698-703 (2010).
- Robbins, J. R., Evanko, S. P., Vogel, K. G. Mechanical Loading and TGF-β Regulate Proteoglycan Synthesis in Tendon. Arch Biochem Biophys. 342 (2), 203-211 (1997).
- Docking, S., Samiric, T., Scase, E., Purdam, C., Cook, J. Relationship between compressive loading and ECM changes in tendons. Muscles Ligaments Tendons J. 3 (1), 7-11 (2013).
- Wang, X., et al. Aberrant TGF-β activation in bone tendon insertion induces enthesopathy-like disease. J Clin Invest. 128 (2), 846-860 (2018).
- Cong, G. T., et al. Evaluating the role of subacromial impingement in rotator cuff tendinopathy: Development and analysis of a novel murine model. J Orthop Res. 36 (10), 2780-2788 (2018).
- Liu, Y., et al. Evaluating the role of subacromial impingement in rotator cuff tendinopathy: development and analysis of a novel rat model. J Shoulder Elbow Surg. 31 (9), 1898-1908 (2022).
- Majima, T., et al. Compressive compared with tensile loading of medial collateral ligament scar in vitro uniquely influences mRNA levels for aggrecan, collagen type II, and collagenase. J Orthop Res. 18 (4), 524-531 (2000).
- Hopkins, C., et al. Critical review on the socio-economic impact of tendinopathy. Asia Pac J Sports Med, Arthrosc, Rehabil Technol. 4, 9-20 (2016).
- Scott, A., Ashe, M. C. Common tendinopathies in the upper and lower extremities. Curr Sports Med Rep. 5 (5), 233-241 (2006).
- Maffulli, N., Wong, J., Almekinders, L. C. Types and epidemiology of tendinopathy. Clin Sports Med. 22 (4), 675-692 (2003).
- Bah, I., et al. Tensile mechanical changes in the Achilles tendon due to Insertional Achilles tendinopathy. J Mech Behav Biomed Mater. 112, 104031 (2020).
- Maffulli, N., Reaper, J., Ewen, S. W. B., Waterston, S. W., Barrass, V. Chondral Metaplasia in Calcific Insertional Tendinopathy of the Achilles Tendon. Clin J Sport Med. 16 (4), 329-334 (2006).
- Corps, A. N., et al. Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatology (Oxford). 45 (3), 291-294 (2006).
- Scott, A., et al. Increased versican content is associated with tendinosis pathology in the patellar tendon of athletes with jumper's knee. Scand J Med Sci Sports. 18 (4), 427-435 (2008).
- Attia, M., et al. Greater glycosaminoglycan content in human patellar tendon biopsies is associated with more pain and a lower VISA score. Br J Sports Med. 48 (6), 469-475 (2014).
- Kujala, U. M., Sarna, S., Kaprio, J. Cumulative Incidence of Achilles Tendon Rupture and Tendinopathy in Male Former Elite Athletes. Clin J Sport Med. 15 (3), 133-135 (2005).
- Corps, A. N., et al. Changes in matrix protein biochemistry and the expression of mRNA encoding matrix proteins and metalloproteinases in posterior tibialis tendinopathy. Ann Rheum Dis. 71 (5), 746-752 (2012).
- Neer, C. S. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 54 (1), 41-50 (1972).
- Bigliani, L. U., Ticker, J. B., Flatow, E. L., Soslowsky, L. J., Mow, V. C. The relationship of acromial architecture to rotator cuff disease. Clin Sports Med. 10 (4), 823-838 (1991).
- Chimenti, R. L., Cychosz, C. C., Hall, M. M., Phisitkul, P. Current Concepts Review Update Insertional Achilles Tendinopathy. Foot Ankle Int. 38 (10), 1160-1169 (2017).
- Nicholson, C. W., Berlet, G. C., Lee, T. H. Prediction of the Success of Nonoperative Treatment of Insertional Achilles Tendinosis Based on MRI. Foot Ankle Int. 28 (4), 472-477 (2007).
- Lohrer, H., David, S., Nauck, T. Surgical treatment for achilles tendinopathy - a systematic review. BMC musculoskelet disord. 17 (1), 207 (2016).
- McGarvey, W. C., Palumbo, R. C., Baxter, D. E., Leibman, B. D. Insertional Achilles Tendinosis: Surgical Treatment Through a Central Tendon Splitting Approach. Foot Ankle Int. 23 (1), 19-25 (2002).
- Maffulli, N., et al. Surgery for chronic Achilles tendinopathy produces worse results in women. Disabil Rehabil. 30 (20-22), 1714-1720 (1714).
- Wunderli, S. L., Blache, U., Snedeker, J. G. Tendon explant models for physiologically relevant in vitro study of tissue biology - a perspective. Connect Tissue Res. 61 (3-4), 262-277 (2020).
- Dyment, N. A., et al. A brief history of tendon and ligament bioreactors: Impact and future prospects. J Orthop Res. 38 (11), 2318-2330 (2020).
- Screen, H. R. C., Berk, D. E., Kadler, K. E., Ramirez, F., Young, M. F. Tendon Functional Extracellular Matrix. J Orthop Res. 33 (6), 793-799 (2015).
- Theobald, P., et al. The functional anatomy of Kager's fat pad in relation to retrocalcaneal problems and other hindfoot disorders. J Anat. 208 (1), 91-97 (2006).
- Ghazzawi, A., Theobald, P., Pugh, N., Byrne, C., Nokes, L. Quantifying the motion of Kager's fat pad. J Orthop Res. 27 (11), 1457-1460 (2009).
- Malagelada, F., et al. Pressure changes in the Kager fat pad at the extremes of ankle motion suggest a potential role in Achilles tendinopathy. Knee Surg Sports Traumatol Arthrosc. 28 (1), 148-154 (2020).
- Shaw, H. M., Benjamin, M. Structure-function relationships of entheses in relation to mechanical load and exercise. Scand J Med Sci Sports. 17 (4), 303-315 (2007).
- Soslowsky, L. J., et al. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng. 30 (8), 1057-1063 (2002).
- Schneeberger, A. G., Nyffeler, R. W., Gerber, C. Structural changes of the rotator cuff caused by experimental subacromial impingement in the rat. J Shoulder Elbow Surg. 7 (4), 375-380 (1998).
- Croen, B. J., et al. Chronic subacromial impingement leads to supraspinatus muscle functional and morphological changes: Evaluation in a murine model. J Orthop Res. 39 (10), 2243-2251 (2021).
- Andarawis-Puri, N., Flatow, E. L. Tendon fatigue in response to mechanical loading. J Musculoskelet Neuronal Interact. 11 (2), 106-114 (2011).
- Gains, C. C., Giannapoulos, A., Zamboulis, D. E., Lopez-Tremoleda, J., Screen, H. R. C. Development and application of a novel in vivo overload model of the Achilles tendon in rat. J Biomech. 151, 111546 (2023).
- Williamson, P. M., et al. A passive ankle dorsiflexion testing system to assess mechanobiological and structural response to cyclic loading in rat Achilles tendon. J Biomech. 156, 111664 (2023).
- Pedaprolu, K., Szczesny, S. E. A Novel, Open-Source, Low-Cost Bioreactor for Load-Controlled Cyclic Loading of Tendon Explants. J Biomech Eng. 144 (8), 084505 (2022).
- Orishimo, K. F., et al. Effect of Knee Flexion Angle on Achilles Tendon Force and Ankle Joint Plantarflexion Moment During Passive Dorsiflexion. J Foot Ankle Surg. 47 (1), 34-39 (2008).
- Liu, C. L., et al. Influence of different knee and ankle ranges of motion on the elasticity of triceps surae muscles, Achilles tendon, and plantar fascia. Sci Rep. 10 (1), 6643 (2020).
- Cruz-Montecinos, C., et al. Soleus muscle and Achilles tendon compressive stiffness is related to knee and ankle positioning. J Electromyogr Kinesiol. 66, 102698 (2022).
- Connizzo, B. K., Grodzinsky, A. J. Lose-dose administration of dexamethasone is beneficial in preventing secondary tendon damage in a stress-deprived joint injury explant model. J Orthop Res. 38 (1), 139-149 (2020).
- Wunderli, S. L., et al. Tendon response to matrix unloading is determined by the patho-physiological niche. Matrix Biol. 89, 11-26 (2020).
- Yabusaki, K., et al. A Novel Quantitative Approach for Eliminating Sample-To-Sample Variation Using a Hue Saturation Value Analysis Program. PloS one. 9 (3), e89627 (2014).
- Gao, J., Messner, K., Ralphs, J. R., Benjamin, M. An immunohistochemical study of enthesis development in the medial collateral ligament of the rat knee joint. Anat Embryol. 194 (4), 399-406 (1996).
- Han, S. K., Wouters, W. A. J., Clark, A., Herzog, W. Mechanically induced calcium signaling in chondrocytes in situ. J Orthop Res. 30 (3), 475-481 (2012).
- Han, W., et al. Impact of cellular microenvironment and mechanical perturbation on calcium signalling in meniscus fibrochondrocytes. Eur Cell Mater. 27, 321-331 (2014).
- Rossetti, L., et al. The microstructure and micromechanics of the tendon-bone insertion. Nat Mater. 16 (6), 664-670 (2017).
- Sartori, J., Köhring, S., Witte, H., Fischer, M. S., Löffler, M. Three-dimensional imaging of the fibrous microstructure of Achilles tendon entheses in Mus musculus. J Anat. 233 (3), 370-380 (2018).
- Eliasberg, C. D., et al. Identification of Inflammatory Mediators in Tendinopathy Using a Murine Subacromial Impingement Model. J Orthop Res. 37 (12), 2575-2582 (2019).
- Zhang, Y., et al. Expression of alarmins in a murine rotator cuff tendinopathy model. J Orthop Res. 38 (11), 2513-2520 (2020).
- Zhang, X., et al. Assessment of Mitochondrial Dysfunction in a Murine Model of Supraspinatus Tendinopathy. J Bone Joint Surg. Am. 103 (2), 174-183 (2021).
- Liu, Y., et al. The role of Indian Hedgehog Signaling in tendon response to subacromial impingement: evaluation using a mouse model. Am J Sports Med. 50 (2), 362-370 (2022).
- Wang, T., et al. Load-induced regulation of tendon homeostasis by SPARC, a genetic predisposition factor for tendon and ligament injuries. Sci Transl Med. 13 (582), eabe5738 (2021).
- Passini, F. S., et al. Shear-stress sensing by PIEZO1 regulates tendon stiffness in rodents and influences jumping performance in humans. Nat Biomed Eng. 5 (12), 1457-1471 (2021).
- Jones, D. L., et al. Mechanoepigenetic regulation of extracellular matrix homeostasis via Yap and Taz. Proc Natl Acad Sci U S A. 120 (22), e2211947120 (2023).
- Connizzo, B. K., Grodzinsky, A. J. Release of pro-inflammatory cytokines from muscle and bone causes tenocyte death in a novel rotator cuff in vitro explant culture model. Connect Tissue Res. 59 (5), 423-436 (2018).
- Rees, S. G., et al. Catabolism of aggrecan, decorin and biglycan in tendon. Biochem J. 350 (Pt 1), 181-188 (2000).
- Samiric, T., Ilic, M. Z., Handley, C. J. Large aggregating and small leucine-rich proteoglycans are degraded by different pathways and at different rates in tendon. Eur J Biochem. 271 (17), 3612-3620 (2004).
- Rees, S. G., Curtis, C. L., Dent, C. M., Caterson, B. Catabolism of aggrecan proteoglycan aggregate components in short-term explant cultures of tendon. Matrix Biol. 24 (3), 219-231 (2005).
- Taye, N., Karoulias, S. Z., Hubmacher, D. The "other" 15-40%: The Role of Non-Collagenous Extracellular Matrix Proteins and Minor Collagens in Tendon. J Orthop Res. 38 (1), 23-35 (2020).
- Carvalho, H. F., Felisbino, S. L. The development of the pressure-bearing tendon of the bullfrog, Rana catesbeiana. Anat Embryol. 200 (1), 55-64 (1999).
- Carvalho, H. F., Felisbino, S. L., Covizi, D. Z., Della Colleta, H. H., Gomes, L. Structure and proteoglycan composition of specialized regions of the elastic tendon of the chicken wing. Cell Tissue Res. 300 (3), 435-446 (2000).
- van Sterkenburg, M. N., Kerkhoffs, G. M., Kleipool, R. P., Niek van Dijk, C. The plantaris tendon and a potential role in mid-portion Achilles tendinopathy: an observational anatomical study. J Anat. 218 (3), 336-341 (2011).
- Lee, A. H., Elliott, D. M. Comparative multi-scale hierarchical structure of the tail, plantaris, and Achilles tendons in the rat. J Anat. 234 (2), 252-262 (2019).
- Lee, A. H., Elliott, D. M. Multi-Scale Loading and Damage Mechanisms of Plantaris and Rat Tail Tendons. J Orthop Res. 37 (8), 1827-1837 (2019).
- Fan, H. M., Shrestha, L., Guo, Y., Tao, H. R., Sun, Y. L. The twisted structure of the rat Achilles tendon. J Anat. 239 (5), 1134-1140 (2021).
- Cutlip, R. G., Stauber, W. T., Willison, R. H., McIntosh, T. A., Means, K. H. Dynamometer for rat plantar flexor muscles in vivo. Med Biol Eng Comput. 35 (5), 540-543 (1997).
- Rijkelijkhuizen, J. M., Baan, G. C., de Haan, A., de Ruiter, C. J., Huijing, P. A. Extramuscular myofascial force transmission for in situ rat medial gastrocnemius and plantaris muscles in progressive stages of dissection. J Exp Biol. 208 (Pt 1), 129-140 (2005).
- Saxena, A., Bareither, D. Magnetic Resonance and Cadaveric Findings of the Incidence of Plantaris Tendon. Foot Ankle Int. 21 (7), 570-572 (2000).
- dos Santos, M. A., Bertelli, J. A., Kechele, P. R., Duarte, H. Anatomical study of the plantaris tendon: reliability as a tendo-osseous graft. Surg Radiol Anat. 31 (1), 59-61 (2009).
- Sartori, J., Köhring, S., Bruns, S., Moosmann, J., Hammel, J. U. Gaining Insight into the Deformation of Achilles Tendon Entheses in Mice. Adv Eng Mater. 23 (11), 2100085 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved