A subscription to JoVE is required to view this content. Sign in or start your free trial.
Visualizing Intracellular Sialylation with Click Chemistry and Expansion Microscopy
In This Article
Summary
Here, we propose a simple protocol combining metabolic oligosaccharide engineering, click chemistry, and expansion microscopy that allows bioimaging of intracellular sialylated N-glycoproteins with improved resolution using routine microscopy equipment.
Abstract
Metabolic labeling techniques allow the incorporation of bioorthogonal reporters into glycans, enabling the targeted bioconjugation of molecular dyes within cells through click and bioorthogonal chemistry. Metabolic oligosaccharide engineering (MOE) has attracted considerable interest due to the essential role of glycosylation in numerous biological processes that involve molecular recognition and its impact on pathologies ranging from cancer to genetic disorders to viral and bacterial infections.
Although MOE is better known for the detection of cell surface glycoconjugates, it is also a very important methodology for the study of intracellular glycans in physiological and pathological contexts. Such studies greatly benefit from high spatial resolution. However, super-resolution microscopy is not readily available in most laboratories and poses challenges for daily implementation. Expansion microscopy is a recent alternative that enhances the resolution of microscopy by physically enlarging biological specimens labeled with fluorescent markers. By embedding the sample in a swellable gel and causing it to expand isotropically through chemical treatment, subcellular structures can be visualized with enhanced precision and resolution without the need for super-resolution techniques.
In this work, we illustrate the capacity of expansion microscopy to visualize intracellular sialylated glycans through the combined use of MOE and click chemistry. Specifically, we propose a procedure for bioorthogonal labeling and expansion microscopy that employs a reporter targeting sialylation, which may be associated with immunofluorescence for co-localization studies. This protocol enables localization studies of sialoconjugate biosynthesis, intracellular trafficking, and recycling.
Introduction
Fluorescence microscopy, while widely used for labeling and visualizing specific molecules within cells, is inherently limited in resolution by Abbe's diffraction limit of light1, which restricts the ability to distinguish between objects closer than approximately 200-250 nm. This limitation arises from the wave nature of light and the numerical aperture of the microscope's objective lens, introducing a challenge when imaging subcellular structures. Overcoming these limitations provides better insights into certain biological processes at a nanometric scale.
To surpass the diffraction limit of light, super-resolution microscopy techniques such as STORM (Stochastic Optical Reconstruction Microscopy) and STED (Stimulated Emission Depletion) have been developed2,3. STORM relies on the stochastic activation of fluorophores, allowing only a sparse subset to be imaged at any given time. This enables the precise localization of individual fluorophores, which allows the reconstruction of a high-resolution image. STED, on the other hand, improves resolution by using a depletion laser to selectively quench fluorescence around the periphery of the excitation spot, effectively narrowing the point spread function.
These approaches contrast with widefield or confocal microscopy, where all fluorophores are simultaneously detected, resulting in an image that combines all diffraction patterns and prevents the distinction between close-by individual fluorophores, leading to a loss of resolution. However, these super-resolution methods require very specific light sources, equipment, sample preparation, and/or fluorophores, making these technologies costly, difficult to access in most laboratories, and challenging to implement in routine experiments. These constraints have led the scientific community to search for alternative solutions to achieve higher resolution, that would be compatible with readily available microscopy equipment and routine staining protocols. In 2015, a method to circumvent the limitations of optical microscopy by physically expanding the sample was developed by Boyden and co-workers, called Expansion Microscopy (ExM)4.
ExM is a three-step method that provides nanoscale details of biological samples without surpassing the diffraction limit of light (Figure 1). Instead, it uses conventional diffraction-limited microscopes to image samples that have been physically magnified in an isotropic manner. The first step, called gelation, consists of embedding the biological sample, typically fixed cells or tissues, in a swellable polyelectrolyte hydrogel based on sodium acrylate and acrylamide. The biological sample then undergoes enzymatic treatment to partially degrade certain components such as proteins or membranes, and break down dense cellular structures to ensure cells can expand uniformly. This step, called digestion, helps achieve this by homogenizing the sample's structural components with regard to mechanical properties, preventing differential expansion that could lead to distortion of the sample. Finally, in the last step, called expansion, the hydrogel-embedded and digested sample is placed in deionized water, causing it to swell. This technology results in the sample expanding by a linear magnification factor of approximately 4-5x in every dimension, allowing for the visualization of fine cellular details using standard microscopy techniques. Given the isotropic nature of the expansion, the biological sample enlarged within the gel matrix retains its three-dimensional geometrical details and the spatial relationships between its various structural components. Spatial information is, therefore, preserved upon swelling, while the distance between the gel-anchored fluorescent labels or biomolecules increases uniformly in all directions. This allows for better separation of signals, resulting in enhanced resolution.
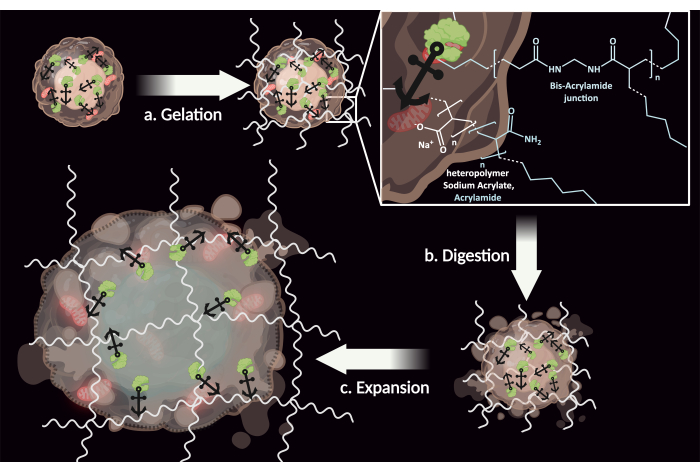
Figure 1: Overview of the ExM Protocol. (a) Gelation: The biological sample is anchored and embedded within a swellable polyelectrolyte hydrogel. (b) Digestion: The mechanical properties of the sample are homogenized through the use of enzymes and detergents, breaking down proteins and lipid membranes that would otherwise restrict the expansion and create distortions. (c) Expansion: The hydrogel is immersed in deionized water, causing it to expand isotropically. Please click here to view a larger version of this figure.
Since 2015, ExM has seen significant technological improvements in preserving spatial information. In particular, various molecular anchors have been designed to covalently attach biomolecules directly to the swellable hydrogel5,6. In this protocol, we used N-acryloxysuccinimide (NAS), which reacts with the free amine groups of biomolecules (typically, lysine residues or N-terminal positions of proteins) to bioconjugate an acryloyl group to the cell's proteins, including glycoproteins. This acryloyl group then reacts with other monomers through cross-linking reactions during gelation, anchoring these biomolecules directly to the polymer.
ExM is compatible with a wide range of labeling methods, including Metabolic Oligosaccharide Engineering (MOE). MOE is a powerful tool that enables the labeling of glycans by incorporating analogs of metabolic precursors equipped with a bioorthogonal chemical handle7. These analogs, known as chemical reporters, integrate into metabolic pathways without causing toxicity. In the MOE approach, reporter monosaccharides are metabolically processed into activated nucleotide sugars and then transferred to nascent glycoconjugates. Our primary focus is on the study of sialylation, particularly the intracellular dynamics and trafficking of sialylated N-glycoproteins, which is crucial in the context of health and disease due to its roles in cell-cell interactions, immune regulation, and development. Abnormal sialylation is implicated in diseases like cancer8,9,10,11, infectious diseases12, and genetic disorders13,14,15, making it a key target for therapeutic development and biomarker discovery. Understanding sialylation enhances insights into glycobiology and disease mechanisms.
Sialylation can be probed using MOE with analogs of N-acetylneuraminic acid (Neu5Ac), the most abundant sialic acid in humans, or with analogs of N-acetylmannosamine (ManNAc), a metabolic precursor of Neu5Ac, bearing a bioorthogonal handle8,16. ManNAc is converted into Neu5Ac in the cytosol, then activated into cytidine-5′-monophospho-N-neuraminic acid (CMP-Neu5Ac) in the nucleus. Once activated into a nucleotide sugar, sialyl transferases in the Golgi apparatus transfer Neu5Ac units to the terminal positions of growing glycan chains (Figure 2). Following the metabolic incorporation of unnatural ManNAc derivatives, the tagged sialylated glycans can be covalently linked to a fluorophore bearing a reactive group complementary to the reporter handle through bioorthogonal click chemistry. This allows for the direct observation of glycoconjugates in vivo or ex vivo.
For most monosaccharide reporters, a peracetylated form is required to cross the plasma membrane via passive diffusion. However, both peracetylated and unprotected reporters have been shown to efficiently probe sialylation16. Unprotected sialylation reporters are able to enter cells by active transport mechanisms, namely pinocytosis for Neu5Ac analogs, and a yet unidentified transporter for ManNAc. While unprotected sugars require a higher concentration (typically 100-500 µM) to achieve comparable effects, once inside the cell, they can directly enter the sialic acid metabolic pathway. In contrast, peracetylated sugars need to be fully deacetylated by intracellular non-specific esterases before becoming metabolically active. Although they can be used at lower concentrations (typically 10-50 µM), incomplete deacetylation may interfere with enzyme activity or lead to the incorporation of partially acetylated sialic acid analogs, potentially skewing downstream analysis. Additionally, the release of acetic acid may affect the pH locally, potentially impacting cellular function. Chen and colleagues have also demonstrated that per-O-acetylated sugars react with free cysteine residues in proteins via a non-enzymatic mechanism, leading to off-target incorporation and increased non-specific signal17,18. In the present protocol, we therefore employ unprotected ManNAc reporters.
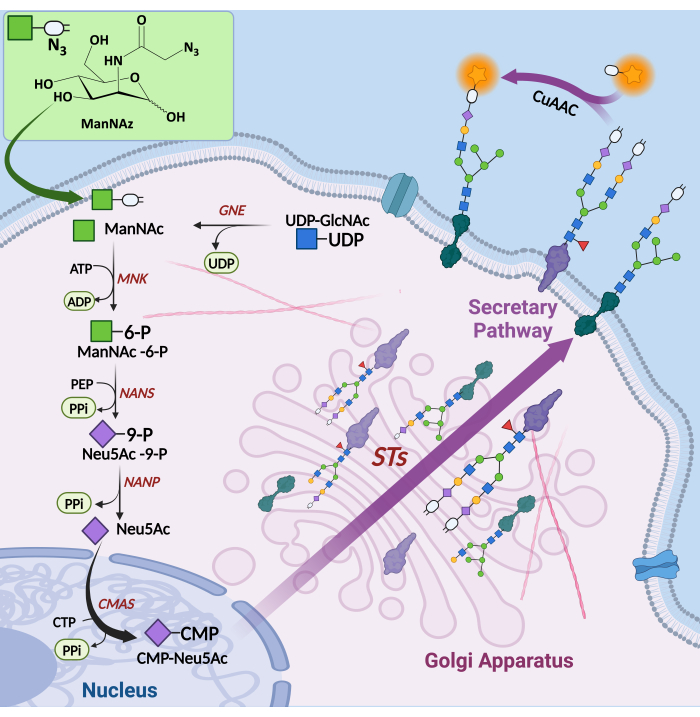
Figure 2: Metabolic oligosaccharide engineering and labeling of sialic acids. UDP-GlcNAc is converted to ManNAc by UDP-GlcNAc 2-epimerase domain of GNE/MNK in the cytosol. ManNAc is then phosphorylated in the cytosol by ManNAc 6-kinase domain of GNE/MNK to form ManNAc-6-phosphate. N-acetylneuraminate synthase catalyzes the condensation of ManNAc-6-P with phosphoenolpyruvate to produce Neu5Ac-9-phosphate, which is subsequently dephosphorylated by sialic acid phosphatase to yield Neu5Ac. Neu5Ac can also be supplied by the salvage pathway via endocytosis and lysosomal recycling8. After transport to the nucleus, it is converted to CMP-Neu5Ac by CMP-sialic acid synthetase. In the Golgi apparatus, CMP-NeuAc is the substrate of sialyltransferases that introduce a Neu5Ac moiety at the terminal positions of glycans on maturing glycoconjugates, which are eventually expressed at the cell membrane or secreted. ManNAz chemical reporters bearing a bioorthogonal handle can penetrate the cell through an unidentified active transporter and enter the metabolic pathway. The tagged Neu5Az units incorporated in glycans after biosynthesis are then labeled through CuAAC-mediated conjugation of a fluorescent probe. Abbreviations: NAc = N-acetyl; UDP-GlcNAc = Uridine diphosphate N-acetylglucosamine; ManNAc = N-acetylmannosamine; GNE = UDP-GlcNAc 2-epimerase; MNK = ManNAc 6-kinase; ManNAc-6-P = ManNAc-6-phosphate; NANS = N-acetylneuraminate synthase; PEP = phosphoenolpyruvate; Neu5Ac = N-acetylneuraminic acid; Neu5Ac-9-P = Neu5Ac-9-phosphate; NANP = sialic acid phosphatase; CMP = cytidine-5′-monophosphate; CMAS = CMP-sialic acid synthetase; STs = sialyltransferases; ManNAz = N-azidoacetylmannosamine; Neu5Az = N-azidoacetylneuraminic acid. Please click here to view a larger version of this figure.
Among the possible bioorthogonal reactions, we focused our interest on the Copper-catalyzed Azide-Alkyne Cycloaddition (CuAAC)16,19. According to our experience, CuAAC is the best option for labeling intracellular glycans in fixed cells. This reaction is well-established, thoroughly studied in bioorthogonal chemistry, and has been standardized for use in complex biological environments, providing a solid foundation of knowledge and optimized protocols. Its fast kinetics indeed offer high reaction efficiency and specificity, and it involves azide and alkyne groups that are easy to synthesize, stable, absent from living systems, and inert toward native biomolecules, making it ideal for fixed-cell applications where copper toxicity is not an issue. Strain-promoted Alkyne-Azide Cycloaddition (SPAAC)20,21, while avoiding copper toxicity, has slower kinetics and bulkier probes, which tends to lead to higher background signal and decreased signal-to-noise ratio for intracellular applications due to hydrophobic trapping, whereas Inverse Electron Demand Diels-Alder (IEDDA)22,23, though fast and copper-free, involves more complex probe synthesis and requires bulkier reporter groups that are yet to be fully characterized in MOE applications. Since ExM exclusively requires fixed cells, CuAAC offers a robust and efficient solution for bioorthogonal labeling.
This paper presents a protocol for the visualization of intracellular sialylated glycoproteins in cells, combining metabolic labeling, bioorthogonal click chemistry, and expansion microscopy. In the experimental procedures described in this paper, we utilize N-azidoacetylmannosamine (ManNAz) as the chemical reporter, and CuAAC ligation of small organic fluorophores is performed prior to the expansion procedure. Glycoproteins are anchored to the hydrogel with NAS prior to gelation, digestion, and expansion. The MOE labeling of sialic acids can be associated with immunofluorescence approaches for co-localization assessment, as exemplified here with a mouse anti-GM130 primary antibody localized in the cis-Golgi apparatus. This protocol can be applied to cells in their physiological state, or to cells that have been subjected to chemical treatment. To illustrate this, chloroquine was used to inhibit lysosomal function, which affects the processing and trafficking of glycoproteins within cells. Nuclear staining is used as a landmark, not only to localize cells but also as an indicator of the quality of the ExM process. The expansion factor can indeed be measured by comparing the size of the nucleus pre-expansion (preExM) and post-expansion (postExM).
Protocol
1. Cell seeding
NOTE: Carry out the next steps under sterile conditions under a laminar flow hood. This method can be applied to any of the cell lines used in the present work (HeLa, MCF7, primary fibroblasts), or to most adherent cell line models commonly used in research20,24,25.
- Grow cells in DMEM high glucose medium supplemented with 10% fetal bovine serum (FBS) in a T75 flask at 37 °C under a 5% CO2 atmosphere.
- Once the cells have reached full confluency, remove the cell culture medium and wash the cells with 4 mL of PBS.
NOTE: PBS solutions used in this step and all subsequent washing steps must be made sterile by filtration through a 0.2 µm PTFE membrane prior to use. - Incubate the cells for 5 min at 37 °C in 2 mL of 1x trypsin-EDTA solution containing 0.5 g/L of trypsin, to release them from the bottom of the flask.
- Add 8 mL of DMEM high glucose medium containing 10% FBS, and mix thoroughly to make sure every single cell is detached from the bottom of the flask.
- Transfer the cells to a 15 mL conical tube and centrifuge for 5 min at 200 × g. In the meantime, add one coverslip (diameter 12 mm, thickness 130-160 µm) to each well of a 12-well plate.
NOTE: The coverslip thickness should be adjusted to match the requirements of the microscope used in subsequent imaging steps. - Remove the supernatant after centrifugation and resuspend the pellet in 1 mL of cell culture medium.
- Determine the average number of cells/mL by hemocytometry across at least two measurements.
- Dilute the cell suspension to a concentration of 300,000 cells per mL in cell culture medium, and seed 1 mL of the suspension into each well.
- Allow the cells to settle for 24 h in DMEM high glucose medium containing 10% FBS at 37 °C under a 5% CO2 atmosphere before the next steps.
2. Chloroquine treatment (optional)
NOTE: Steps 2.1-2.8 illustrate how to use the protocol on cells that are treated with an external reagent (inhibitor, effector, drug), using chloroquine as an example. Skip these steps for cells untreated with external reagents.
- Weigh the correct amount of chloroquine diphosphate salt (CQ) to prepare a sufficient volume of a 10 mM stock solution.
NOTE: Carry out the next steps under sterile conditions under a laminar flow hood. - Solubilize the solid CQ in the desired amount of deionized water for a final concentration of 10 mM.
- Filter the solution through a 0.2 µm filter.
- Aliquot 1 mL of the 10 mM stock solution in 1.5 mL microcentrifuge tubes and store them at -20 °C.
- Wash the cell sample prepared in step 1.9 with 1 mL of PBS.
- Add 100 µL of 10 mM CQ in 9.9 mL of DMEM high glucose medium containing 10% FBS, for a final concentration of 100 µM of CQ.
- Incubate cells for 8 h in 1 mL of a medium supplemented with 100 µM of CQ at 37 °C under a 5% CO2 atmosphere.
- Wash the cells 3x with 1 mL of PBS.
3. Metabolic oligosaccharide engineering
- Prepare a 5 mM stock solution of ManNAz (or ManNAc for the negative control condition) in DMEM high glucose medium containing 10% FBS.
NOTE: This stock solution should be of sufficient volume such that each well can be treated with 1 mL of sugar analog solution. Carry out the next steps under sterile conditions under a laminar flow hood. - Filter the solution through a 0.2 µm filter and store in 1 mL aliquots at -20 °C until required.
- Prepare a medium supplemented with 500 µM of ManNAz or ManNAc by diluting a 1 mL stock solution aliquot prepared in step 3.2 with 9 mL of DMEM high glucose medium containing 10% FBS.
- Incubate the cells prepared in step 1.9 (for experiments on untreated cells) or in step 2.8 (for experiments on CQ-treated cells) in 1 mL of the medium supplemented with 500 µM ManNAz (or 500 µM ManNAc for the control group) for 24 h at 37 °C under a 5% CO2 atmosphere.
4. Fixation and permeabilization
NOTE: All these steps are carried out under a fume hood.
- Wash the samples with 1 mL of PBS.
- Fix the cells on each coverslip by adding 500 µL of 5% paraformaldehyde. Let it rest for 15 min at room temperature.
- Wash 3x with 1 mL of PBS.
- Prepare a custom humid chamber using a sufficiently large opaque box (e.g., a polystyrene box) with a lid. Place a wet piece of blotting paper at the bottom of the box, and then lay a layer of parafilm on top of it.
NOTE: The humid chamber should completely protect the sample from outside light sources when closed. - Place the coverslips on the parafilm, cells facing up.
NOTE: Label the parafilm with the name or reference of the samples in advance. - Carry out cell permeabilization using 200 µL of 0.5% (v/v) Triton X-100 in PBS for 15 min at room temperature.
- Wash 3x with 200 µL of PBS.
5. Fluorescence labeling
- CuAAC labeling of tagged sialylated glycans
NOTE: The CuAAC reaction buffer must be freshly prepared prior to use, and the reactants should be added in the specified order, with sodium ascorbate being added last. In oxygenated aqueous solutions, Cu(I) ions formed by the reduction of Cu(II) with ascorbate will gradually re-oxidize to Cu(II), leading to a decrease in ascorbate concentration over time. To maintain result reproducibility, the buffer should not be stored for more than a few days at 4 °C and is preferably used on the same day.- To label the azide-modified sialylated glycans engineered in section 3, prepare a CuAAC reaction buffer consisting of 5 µM AlexaFluor 488 alkyne (AF488Alk), 150 µM CuSO4, 300 µM 2-(4-((bis((1-(tert-butyl)-1H-1,2,3-triazol-4-yl)methyl)amino)methyl)-1H-1,2,3-triazol-1-yl)acetic acid (BTTAA), 0.1 M K2HPO4, and 2.5 mM sodium ascorbate in deionized water. Prepare enough solution to apply 200 µL to each coverslip.
- Add 200 µL of CuAAC buffer on each coverslip to initiate the reaction. Make sure the sample is homogeneously covered with the drop, and let it react for 45 min at room temperature, protected from the light.
- Wash 3x with 200 µL of PBS to stop the reaction and remove excess probe and reagents.
- Nucleus staining
- For nuclear labeling with Hoechst 33342, dilute a 10 mg/mL stock solution at 1:2000 in PBS.
NOTE: Equivalent nuclear counterstains such as 4',6-diamidino-2-phenylindole (DAPI) may be used to the same effect. - Cover the cells with 200 µL of this solution and incubate for 5-10 min at room temperature, protected from the light.
- Wash 3x with 200 µL of PBS.
- For nuclear labeling with Hoechst 33342, dilute a 10 mg/mL stock solution at 1:2000 in PBS.
- Immunofluorescence staining
- Prepare a Bovine Serum Albumin (BSA) blocking buffer containing 0.1 g of gelatin, 1 g of BSA, 1 mL of FBS, and 49 mL of PBS for 50 mL of buffer.
- Incubate the samples for 1 h at 4 °C in 200 µL of BSA blocking buffer. Protect the sample from light.
- For immunofluorescence staining of the Golgi apparatus using a purified mouse anti-GM130 antibody (epitope recognized: Rat GM130 aa. 869-982 | RRID: AB_398142), dilute the primary antibody at 1:100 in the prepared BSA blocking buffer.
NOTE: This step can be adapted to immunofluorescence staining targeting other organelles by using any relevant primary antibody, in which case the dilution factor should be adapted as per the manufacturer's guidance. - Add 70 µL of the solution containing the antibody to the coverslips and incubate for 1 h at 4 °C, protected from light.
- Wash 3x with 200 µL of PBS.
- Dilute the fluorescent secondary antibody (Alexa Fluor 546 anti-mouse IgG) at 1:600 in BSA blocking buffer and add 100 µL on each coverslip.
- Incubate the samples for 1 h at room temperature, protected from light.
- Wash 3x with 200 µL of PBS.
- Transfer the coverslips to a 6-well plate. Store the samples in 2 mL of PBS at 4 °C for a few days, protected from light.
NOTE: it is advised to always prepare a replicate exclusively for visualization preExM as a reference for comparison purposes before and after expansion and to ensure the labeling pattern has not been distorted after expansion.
6. Preexpansion imaging of samples
NOTE: The following steps are dependent on the bioimaging equipment used and may need to be adjusted to the requirements of the machine. Please follow the rules of the local laboratory or bioimaging platform. Steps 6.1-6.13 here are carried out on a laser scanning confocal microscope.
- Turn on the microscope and ensure that light sources (lasers) are warmed up. Open the bioimaging acquisition software.
- Select the laser excitation wavelengths corresponding to the fluorophores to create the channels (e.g., 405 nm for nuclear staining, 488 nm for AF488Alk-labeled sialylated glycans, and 546 nm for immunofluorescence labeling). Activate the lasers accordingly.
- Place and secure a 32 mm diameter coverslip (130-170 µm thickness) in a holder adapted to the microscope.
- Place the sample prepared in step 5.3.9, cells facing down, on the 32 mm coverslip. Add a drop of deionized water to prevent the sample from drying out.
- Select an appropriate objective lens, here a 63x oil immersion objective with a 1.4 numerical aperture or equivalent.
NOTE: The highest numerical aperture provides the best resolution. When using an oil immersion objective, remember to add a drop of oil between the objective and the coverslip. - Place the holder on the microscope stage above the objective.
- Use brightfield or low laser intensity fluorescence mode to locate an area of interest in the sample.
- Set the focus.
- Visualize the cells in the software in live fluorescence mode. Set the laser intensity for each channel. Start with low laser power to avoid photobleaching. Slowly increase the laser power and adjust the detector gain and offset to amplify the signal without introducing excessive noise as needed to visualize the fluorescence clearly without overexposure.
NOTE: Parameters should be adjusted to minimize both background noise and avoid signal saturation. It is crucial to maintain consistent settings across all samples in a series of experiments, including negative controls. - Set image parameters such as pinhole size (e.g., 1 Airy Unit), scan speed (e.g., 8), zoom factor (e.g., 1), range of brightness values (e.g., 16 bits), and averaging number (e.g., 2), mode (e.g., line), and method (e.g., mean) as needed to reach the desired observation.
NOTE: These values are system- and sample-dependent and should be optimized prior to acquisition. Pinhole size depends on the numerical aperture, magnification, and wavelength: decreasing it may improve resolution at the expense of signal-to-noise ratio. It is advised to set scan speed to fast values (corresponding to small dwell time per pixel) to avoid photobleaching. - Perform the acquisition and save data in the desired format with proper metadata for future reference.
- Once the acquisition is over, put the sample back in the 6-well plate by adding a small amount of deionized water to facilitate the transfer with a pair of tweezers.
NOTE: Step 6.12 is only carried out if expansion of this particular sample is needed. Otherwise, dispose of the sample and use other replicates for the following steps.
7. Expansion microscopy protocol
NOTE: The samples are protected as much as possible from the light to avoid photobleaching. The anchoring and gelation steps are carried out under a fume hood.
- Anchoring
- Incubate the samples with N-acryloylsuccinimide (NAS) at a concentration of 3.2 mg/mL in PBS on a mechanical shaker for 1 h at room temperature. Prepare a sufficient amount of solution to add 500 µL of solution per well (stock solution: 16 mg/mL NAS in DMSO).
NOTE: NAS reacts with the lysine residues and N-termini of proteins to graft an acryloyl group, enabling the proteins to anchor to the hydrogel in subsequent steps. - Wash 3x with 1 mL of PBS.
- Incubate the samples with N-acryloylsuccinimide (NAS) at a concentration of 3.2 mg/mL in PBS on a mechanical shaker for 1 h at room temperature. Prepare a sufficient amount of solution to add 500 µL of solution per well (stock solution: 16 mg/mL NAS in DMSO).
- Gelation
- Prepare a monomer solution composed as follows: in 50 mL of 1x PBS (pH 7.4), add 5.843 g of NaCl, 1.25 g of acrylamide, 0.075 g of bis-acrylamide, and 4.313 g of sodium acrylate. Prepare enough solution to apply 70 µL to each coverslip. Freeze 1 mL aliquots at least 24 h before gelation.
CAUTION: The monomers are toxic; they must be handled with care with appropriate protection equipment and weighed under a fume hood. - On a piece of parafilm, a 70 µL drop of monomer solution is placed, followed by the addition of 1.4 µL of 10% (v/v) N,N,N′,N′-tetramethylethylenediamine (TMEDA), then 1.4 µL of 10% (w/v) Ammonium persulfate (APS), which are mixed into the drop.
NOTE: The crosslinker reagents must be added in this specific order, TMEDA first, then APS. - Quickly, place the coverslip carefully on the solution with the cells facing down.
- Allow the solution to polymerize for 1 h at room temperature in a humid chamber.
- Prepare a monomer solution composed as follows: in 50 mL of 1x PBS (pH 7.4), add 5.843 g of NaCl, 1.25 g of acrylamide, 0.075 g of bis-acrylamide, and 4.313 g of sodium acrylate. Prepare enough solution to apply 70 µL to each coverslip. Freeze 1 mL aliquots at least 24 h before gelation.
- Digestion
- Prepare a digestion buffer solution composed of 1x TAE buffer, 0.5% (v/v) Triton X-100, 0.8 M guanidine HCl, and 8 U/mL proteinase K.
NOTE: The digestion buffer must be freshly prepared and preincubated for 30 min at 37 °C before use. - Add 1 mL of digestion buffer to the hydrogel formed in step 7.2. Allow the digestion to proceed for 3 h at 37 °C on a mechanical shaker.
- Remove the digestion buffer, and gently wash the gel 3x with 2 mL of deionized water.
NOTE: The hydrogel will slightly expand and detach itself from the cover slip.
- Prepare a digestion buffer solution composed of 1x TAE buffer, 0.5% (v/v) Triton X-100, 0.8 M guanidine HCl, and 8 U/mL proteinase K.
- Expansion
- Leave the hydrogel to expand in 3 mL of deionized water for 2 h. Change the water every 30 min.
8. Post expansion imaging of samples
- Place a 32 mm diameter coverslip (130-170 µm thickness) and secure it in on a holder.
- Cut out a piece of the hydrogel and place it on the cover slip, with the cells at the bottom of the gel.
NOTE: The excised pieces should be large enough to provide adequate surface area for observation but not too large, as this could make handling the gel difficult and increase the risk of breakage (recommended size: 5 to 8 mm). Place enough hydrogel pieces to cover the entire surface of the coverslip; this will help secure the pieces in place and prevent any drift during imaging. In case of drifting issues, precoating the coverslips with poly-D-lysine might prove advantageous6. - Place the holder on the microscope stage above the objective.
- Perform the image acquisition on a Laser Scanning Confocal Microscope with an oil immersion objective as described in step 6.
9. Expansion Factor (EF) calculation in ImageJ
- Open the imaging data in the ImageJ freeware or equivalent.
- If working on multiple channels image, split the channels and work only on the nucleus channel by navigating to Select Image | Color | Split channels.
- To measure the nucleus diameter, highlight the signal corresponding to the nucleus by determining a threshold by clicking on Select Image | Adjust | Threshold…. Choose a method in the default dropdown menu that best fits the image (e.g., Otsu), and adjust the threshold until the shape of the nucleus is clearly cut out from the background.
NOTE: The threshold setting needs to be adjusted for each image as one setting might not fit all images or every sample. - Set the measurement to determine the Feret diameter by clicking on Select Analyze | Set measurements…. From the menu, select Feret diameter and adjust the decimal if necessary.
- To begin the analysis of the image, click on Select Analyze | Analyze particles to set the size of the particles; for the nucleus, choose 5-infinity µm² | Display results | Add to manager | Exclude on edges & Overlay. Start the analysis.
- As the analysis begins, multiple windows will appear. In the window results, look for the maximum Feret diameter of each nucleus in the column Feret. Copy-paste the raw data to a spreadsheet and calculate the average nucleus size for preexpanded and expanded samples.
NOTE: Make sure that the regions of interest (ROIs) corresponding to each nucleus follow the entire periphery of the nucleus. - Calculate the expansion factor (EF) as follows:
EF =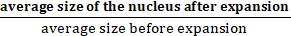
NOTE: The EF factor determines how much the sample has been expanded.
Results
Shown below is the application of the protocol to visualize sialylated glycoproteins in HeLa cells (Figure 3A) and MCF7 cells (Figure 3B), omitting CQ treatment (protocol section 2) and immunofluorescence co-localization staining (protocol step 5.3).
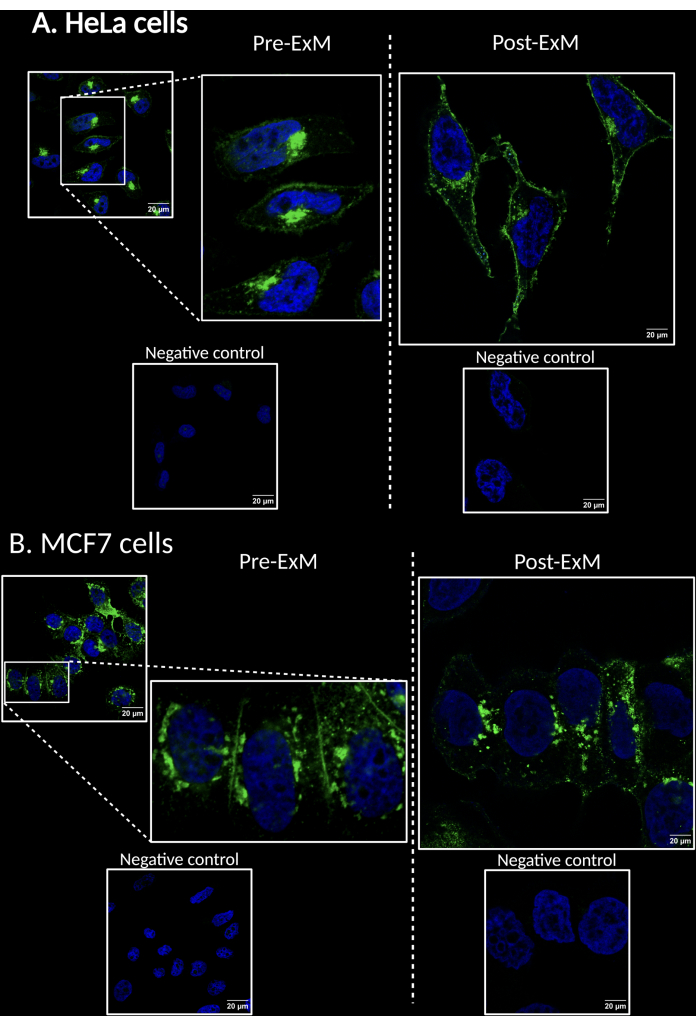
Figure 3: Compar...
Discussion
The present CuAAC labeling protocol does not include aminoguanidine in the reaction buffer. Since it is aimed at visualizing intracellular glycoconjugates, it is performed on cells that are fixed after the metabolic incorporation step, to avoid any cytotoxicity issue and improve uptake of the catalytic system. The use of aminoguanidine is typically recommended for cell-surface labeling of living cells to prevent side reactions between dehydroascorbate and arginine, histidine, and lysine residues of proteins
Disclosures
The authors have no competing financial interests or other conflicts of interest.
Acknowledgements
We thank the TisBio facilities and the PLBS platform for providing the technical environment conducive to achieving this work. This work was supported by grants from the CNRS and the Ministère de l'Enseignement Supérieur et de la Recherche. We would like to thank Dr. François Foulquier, Dr. Zoé Durin, Mrs. Dorothée Vicogne, and Mrs. Céline Schulz for stimulating discussions and for providing us with the Fibroblast 533T cell line and the primary antibody GM130.
Materials
| Name | Company | Catalog Number | Comments |
| (+) Sodium L-ascorbate | Sigma Aldrich | 11140 | |
| 12 well cell culture plate | Corning | 3513 | |
| Acrylamide | Sigma Aldrich | A8887 | |
| Acrylic acid N-hydroxysuccinimide ester | Sigma Aldrich | A8060 | |
| Alexa Fluor 488 alkyne | Jena Bioscience | CLK-1277-5 | |
| Alexa Fluor 546 goat anti-mouse IgG | Invitrogen | A11003 | |
| Amonium persulfate | Sigma Aldrich | 9913 | |
| Bis-Acrylamide | Sigma Aldrich | 146072 | |
| BSA | Sigma Aldrich | A7906 | |
| BTTAA | Jena Bioscience | CLK-067-100 | |
| Centrifugation tube 2 mL | EPPENDORF | 30120094 | |
| Chloroquine diphosphate salt | Sigma Aldrich | C6628 | |
| Conical tube 15 mL | Falcon | 352097 | |
| cover slips 12 mm #1 | epredia | CB00120RA120MNZ0 | |
| cover slips 32 mm #1 | epredia | CB00320RA140MNZ0 | |
| CuSO4 | Sigma Aldrich | 209198 | |
| DMEM high glucose medium | Dutscher | L0104-500 | |
| Dulbecco's Phosphate Buffered Saline (PBS) | Dutscher | L0615-500 | |
| Fetal Bovine Serum | biowest | S1810-500 | |
| Fibroblast 533T | - | - | Collected from healthy individual |
| FIJI ImageJ 2.9.0 | - | - | |
| Gelatin | Bio-RAD | 170-6537 | |
| Guanidine HCl | Sigma Aldrich | 50950 | |
| HeLa cells | ATCC | CCL-2 | |
| Hoechst 33342 | Sigma Aldrich | 14533 | |
| Imaris 10.2 | - | - | |
| K2HPO4 | Euromedex | PB0447-B | Anhydrous |
| LSM 780 Confocal Microscopy | Zeiss | - | |
| MCF7 | ATCC | HTB-22 | |
| N-acetylmannosamine (ManNAc) | BIOSYNTH | MA05269 | |
| NaCl | Carlo Erba | 479687 | |
| N-azidoacetylmannosamine (ManNAz) | BIOSYNTH | MA46002 | |
| Objectif "Plan-Apochromat" 63x/1,4 Oil DIC M27 | Zeiss | 420782-9900-799 | |
| Phosphate Buffered Saline (PBS) 10x | Euromedex | ET330 | |
| Proteinase K | Sigma Aldrich | P2308 | from Tritirachium album |
| purified mouse GM130 antibody | BD Bioscience | 610822 | 50 µg |
| Sodium acrylate | Sigma Aldrich | 408220 | |
| T75 Flask | Corning | 430641 | |
| TEMED | Sigma Aldrich | T9281 | |
| tris Acetate EDTA (TAE) 10x | Euromedex | EU0202-B | |
| Triton X-100 | Sigma Aldrich | X-100 | |
| Trypan Blue | Dutscher | 702630 | |
| Trypsine-EDTA 1x | Dutscher | L0930-100 |
References
- De Souza, N. Light microscopy at the limit. Nat Cell Biol. 11 (Suppl 1) S22 (2009).
- Tam, J., Merino, D. Stochastic optical reconstruction microscopy (STORM) in comparison with stimulated emission depletion (STED) and other imaging methods. J Neurochem. 135, 643-658 (2015).
- Jeong, S., Widengren, J., Lee, J. C. Fluorescent probes for STED optical nanoscopy. Nanomaterials. 12 (1), 21 (2021).
- Chen, F., Tillberg, P. W., Boyden, E. S. Expansion microscopy. Science. 347 (6221), 543548 (2015).
- Kang, S. et al. Expansion microscopy with a thermally adjustable expansion factor using thermoresponsive biospecimen-hydrogel hybrids. ACS Appl Mater Interfaces. 13 (24), 2896228974 (2021).
- Sun, D. et al. Click-ExM enables expansion microscopy for all biomolecules. Nat Methods. 18 (1), 107113 (2020).
- Dube, D., Bertozzi, C. Metabolic oligosaccharide engineering as a tool for glycobiology. Curr Opin Chem Biol. 7 (5), 616625 (2003).
- Scache, J. et al. Switching azide and alkyne tags on bioorthogonal reporters in metabolic labeling of sialylated glycoconjugates: a comparative study. Sci Rep. 12 (1), 22129 (2022).
- Holst, S., Wuhrer, M., Rombouts, Y. Glycosylation characteristics of colorectal cancer. Adv Cancer Res. 126, 203256 (2015).
- Boyaval, F. et al. N-Glycomic signature of stage II colorectal cancer and its association with the tumor microenvironment. Mol Cell Proteomics. 20, 100057 (2021).
- Ferreira, J. A. et al. Protein glycosylation in gastric and colorectal cancers: Toward cancer detection and targeted therapeutics. Cancer Lett. 387, 32-45 (2017).
- Jennings, M. P., Day, C. J., Atack, J. M. How bacteria utilize sialic acid during interactions with the host: snip, snatch, dispatch, match and attach. Microbiology. 168 (3), 001157 (2022).
- Gilormini, P. A. et al. A sequential bioorthogonal dual strategy: ManNAl and SiaNAl as distinct tools to unravel sialic acid metabolic pathways. Chem Commun. 52 (11), 2318-2321 (2016).
- Vanbeselaere, J. et al. Alkynyl monosaccharide analogues as a tool for evaluating Golgi glycosylation efficiency: application to Congenital Disorders of Glycosylation (CDG). Chem Commun. 49 (96), 11293-11295 (2013).
- Gilormini, P. A. et al. Chemical glycomics enrichment: imaging the recycling of sialic acid in living cells. J Inherit Metab Dis. 4 (3), 515523 (2018).
- Rigolot, V., Biot, C., Lion, C. To view your biomolecule, click inside the cell. Angew Chem. 60 (43), 2308423105 (2021).
- Qin, W. et al. Artificial cysteine S-glycosylation induced by per-O-acetylated unnatural monosaccharides during metabolic Glycan Labeling. Angew Chem. 130 (7), 1835-1838 (2018).
- Qin, K., Zhang, H., Zhao, Z., Chen, X. Protein S-glyco-modification through an elimination-addition mechanism. J Am Chem Soc. 142 (20), 9382-9388 (2020).
- Rostovtsev, V. V., Green, L. G., Fokin, V. V., Sharpless, K. B. A. Stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew Chem. 41 (14), 25962599 (2002).
- Agard, N. J., Prescher, J. A., Bertozzi, C. R. A strain-promoted [3 + 2] azide−alkyne cycloaddition for covalent modification of biomolecules in living systems. J Am Chem Soc. 126 (46), 15046-15047 (2004).
- Mbua, N. E. et al. Strain-promoted alkyne-azide cycloadditions (SPAAC) reveal new features of glycoconjugate biosynthesis. ChemBioChem. 12 (12), 19121921 (2011).
- Blackman, M. L., Royzen, M., Fox, J. M. Tetrazine ligation: Fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J Am Chem Soc. 130 (41), 1351813519 (2008).
- Devaraj, N. K., Weissleder, R., Hilderbrand, S. A. Tetrazine-based cycloadditions: Application to pretargeted live cell imaging. Bioconjug Chem. 19 (12), 22972299 (2008).
- Bird, R. E. et al. Bioorthogonal chemistry and its applications. Bioconjug Chem. 32 (12), 2457-2479 (2021).
- Slade, P. G. et al. Identifying the CHO secretome using mucin-type O-linked glycosylation and click-chemistry. J Proteome Res. 11 (12), 61756186 (2012).
- Gambarotto, D., Hamel, V., Guichard, P. Ultrastructure expansion microscopy (U-ExM). Methods Cell Biol. 161, 5781 (2021).
- Gambarotto, D. et al. Imaging cellular ultrastructures using expansion microscopy (U-ExM). Nat. Methods. 16, 71-74 (2019).
- Presolski, S. I., Hong, V. P., Finn, M. Copper-catalyzed azide-alkyne click chemistry for bioconjugation. Curr Protoc Chem Biol. 3 (4), 153-162 (2011).
- Santiago, F., et al. Synthesis and swelling behaviour of poly(sodium acrylate)/sepiolite superabsorbent composites and nanocomposites. Polym Int. 55 (8), 843848 (2006).
- Wen, G., et al. Evaluation of direct grafting strategies via trivalent anchoring for enabling lipid membrane and cytoskeleton staining in expansion microscopy. ACS Nano. 14 (7), 78607867 (2020).
- Wen, G. et al. Current progress in expansion microscopy: Chemical strategies and applications. Chem Rev. 123 (6), 32993323 (2023).
- Drelich, L. et al. Toward high spatially resolved proteomics using expansion microscopy. Anal Chem. 93 (36), 12195-12203 (2021).
- Zwettler, F. U. et al. Molecular resolution imaging by post-labeling expansion single-molecule localization microscopy (Ex-SMLM). Nat Commun. 11 (1), 3388 (2020).
- Liu, J. et al. Expansion microscopy with multifunctional polymer dots. Adv Mater. 33 (25), 2007854 (2021).
- Klimas, A. et al. Magnify is a universal molecular anchoring strategy for expansion microscopy. Nat Biotechnol. 41 (6), 858869 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved