Extracting the Young's Modulus of Native Murine Pulmonary Basement Membranes from Atomic Force Microscopy derived Force Maps
* These authors contributed equally
In This Article
Summary
This protocol visualizes how to prepare cryosections of murine lung tissue, perform atomic force microscopy force map experiments, and analyze the data to determine Young's modulus of the native murine pulmonary basement membrane.
Abstract
Atomic force microscopy (AFM) allows the characterization of the mechanical properties of a sample with a spatial resolution of several tens of nanometers. Because mammalian cells sense and react to the mechanics of their immediate microenvironment, the characterization of biomechanical properties of tissues with high spatial resolution is crucial for understanding various developmental, homeostatic, and pathological processes. The basement membrane (BM), a roughly 100 - 400 nm thin extracellular matrix (ECM) substructure, plays a significant role in tumor progression and metastasis formation. Although determining Young's modulus of such a thin ECM substructure is challenging, biomechanical data of the BM provides fundamental new insights into how the BM affects cell behavior and, in addition, offers valuable diagnostic potential. Here, we present a visualized protocol for assessing BM mechanics in murine lung tissue, which is one of the major organs prone to metastasis. We describe an efficient workflow for determining the Young's modulus of the BM, which is located between the endothelial and epithelial cell layers in lung tissue. The step-by-step instructions comprise murine lung tissue freezing, cryosectioning, and AFM force-map recording on tissue sections. Additionally, we provide a semi-automatic data analysis procedure using the CANTER Processing Toolbox, an in-house developed user-friendly AFM data analysis software. This tool enables automatic loading of recorded force maps, conversion of force versus piezo-extension curves to force versus indentation curves, computation of Young's moduli, and generation of Young's modulus maps. Finally, it shows how to determine and isolate Young's modulus values derived from the pulmonary BM through the use of a spatial filtering tool.
Introduction
AFM force maps have emerged as a widely utilized technique for determining the nanomechanical properties of a diverse range of biological samples with nanometer spatial resolution and piconewton force sensitivity1,2,3,4,5,6,7,8,9,10,11,12. Pioneering research on the application of AFM measurements to biological samples under physiological conditions was conducted by P. Hansma and colleagues13,14,15,16,17,18,19,20. Subsequently, AFM force measurements were employed to assess the mechanical properties of individual molecules21, living cells and their cytoskeleton9,10,22,23,24,25,26, tissues and tissue sections6,8,11,27,28,29,30,31, as well as biological hydrogels3,4,32. In our research, we used AFM to examine the mechanical properties of biological and chemical bonds18,33,34,35,36,37 and to measure the nanomechanical characteristics of individual cells38,39,40. Moreover, measurements of growth plate cartilage8 demonstrated that the collagen architecture and Young's modulus of the extracellular matrix (ECM) direct chondrocyte division and, consequently, the growth direction of the murine growth plate during embryonic development. Furthermore, we investigated the changes associated with cartilage degeneration and osteoarthritis in articular cartilage2,41,42,43,44,45,46,47.
In recent years, numerous AFM investigations have examined the mechanical properties of both healthy and pathological pulmonary tissue48,49,50,51,52. However, these investigations primarily examined overall tissue mechanics, not focusing on specific tissue components such as the BM. The BM constitutes a thin layer (100 nm - 400 nm in humans) of a specialized ECM structure that lines the majority of mammalian organs and tissue structures such as neurons, muscles, adipocyte tissues, and blood vessels. We discovered that the mechanical properties of the murine pulmonary BM play a pivotal role during the metastasis formation in a way that a softer BM correlates with a higher survival probability in breast and kidney cancer patients53. Furthermore, this investigation revealed that the secreted extracellular matrix protein netrin-4 decreases the Young's modulus of the BM through a stoichiometric 1:1 interaction with laminin γ1, a key component present in nearly all laminin networks inside BMs40.
Here, we present the visualization of our developed protocol to determine the Young's modulus (EBM) of the 100 - 200 nm thin murine pulmonary BM. The Young's modulus of the BM is a measure of its stiffness and is defined as the ratio of stress (force per unit area) versus the resulting axial strain (displacement) during a linear elastic deformation54, in this case, a small compression of the BM by the indenting AFM tip. In an AFM experiment, the force and the displacement (indentation depth) can be obtained from the AFM cantilever deflection and the cantilever position, and Young's modulus (the stress-strain-relation) can be extracted from the resulting force versus indentation curve by using a suitable elastic model, which accounts for the geometry of the indenting tip (in this case a modified Hertz Model55). The main steps are shown in Figure 1. Initially, this protocol delineates the methodology for preparing and embedding murine lung tissue samples in OCT compound (see step 1), thereby facilitating the subsequent cryosectioning procedure (step 2) that enables the determination of the BM stiffness utilizing AFM force maps. The cryosectioning technique presented here, which utilizes one-sided and double-sided tape, enables the sectioning of lung tissue without the requirement of fixation and without thawing of the section. The detailed AFM procedure for collecting suitable data to assess BM stiffness is outlined in the third section of the protocol. In the next section of the protocol, we explain how the generated force volumes can be automatically analyzed (step 4.1) using an in-house developed MATLAB-based software, the CANTER Processing Toolbox56. In this step, the Young's modulus is extracted from each recorded force-indentation curve by fitting a modified Hertz model, which is corrected for the indenter geometry, in this case, a four-sided pyramid55. Finally, we describe in steps 4.2 and 4.3 how to apply the Spatial Filtering Tool and the R2 Filtering Tool of the developed toolbox to extract the Young's modulus values specific to the basement membrane (BM) from the comprehensive set of Young's modulus values obtained in the force map containing all tissue compartments of the alveolar wall.
Protocol
All procedures for handling animal samples were approved by the Animal Experimentation Council of the Ministry of Environment and Food of Denmark (permission number 2017-15-0201-01265) according to the Danish Law of Animal Welfare. To demonstrate this protocol, we used a male C57BL/6 mouse at 13 weeks of age.
CAUTION: In the following protocol, several steps require handling of tissue specimens. Always wear adequate gloves and a laboratory coat while handling biological samples.
1. Sample preparation
- Place a freshly harvested mouse lung into a disposable 15 x 15 x 5 mm base mold. Utilizing a 10 mL syringe with a 0.9 mm needle, carefully inject a 1:1 (volume-to-volume) ratio of optimal cutting temperature (OCT) medium to phosphate buffered saline (PBS) solution into the sample while simultaneously retracting the needle. Conduct the injection at room temperature while holding the sample securely with a small surgical tweezer. The injection is considered complete when the sample becomes swollen, and a noticeable amount of oozing is observed.
- Fill pure OCT in the mold until the specimen is fully immersed. To ensure complete freezing of the entire lung, store the sample at a temperature of -80 °C for at least 4 h.
NOTE: At this point, the sample can be stored for at least 2 years at -80 °C. Based on the manufacturer's specifications for the OCT, the storage time of embedded tissue is over 10 years. It is generally recommended to wrap samples that need to be stored for periods exceeding 4 months in a transparent film to prevent moisture from being absorbed into the sample.
2. Cryosectioning of embedded lung specimen
- Regulate the temperature of the cryotome's chamber to -17 °C, as this is the optimal cutting temperature for lung tissue samples. Set the temperature of the cutting stage unit to -22 °C, if this feature is available. This serves to ensure that the optimal cutting temperature is maintained on the sample surface throughout the entire sectioning process.
- Prepare 40 microscope slides using double-sided adhesive tape that is carefully placed in the middle of the glass slide before rolling a 15 mL centrifuge tube over the tape to ensure firm and bubble-free adhesion. Ensure the length of the double-sided adhesive tape is roughly equivalent to the width of the OCT block containing the sample. Microscope slides with a frosted edge allow labeling with a pencil containing sample information and the section number.
- Insert the prepared microscope slides into a slide box. Subsequently, place the box holding the slides within the cryotome chamber in order to chill them.
NOTE: Chilling the slides prevents the occurrence of a freeze-thaw cycle when picking up the sections, which would compromise the mechanical properties of the sample. - Prepare multiple single-sided adhesive tapes, each measuring roughly the same size as the frozen sample block.
- Fill the inner two rings of the sample holder with OCT medium. Position the sample within the OCT medium on the sample holder, ensuring it is as level as possible. Subsequently, place the sample holder for approximately 10 min within the cryotome chamber until the OCT medium has solidified completely and the sample is firmly secured to the holder.
- Install the holder bearing the sample onto the cutting stage unit of the cryotome. Adjust all the essential components of the cryotome, such as the inclination of the cutting stage and the position of the blade, according to the user manual of the device to establish a cutting plane that is aligned parallel to the surface of the OCT block.
- Undertake initial trimming of the sample by adjusting the sectioning thickness to 50 µm and commence cutting until reaching the desired location within the sample (usually about 500 µm inside the lung specimen from its surface) for the collection of tissue slides. Adjust the sectioning thickness of the cryotome to 15 µm, which corresponds to the desired thickness of the tissue sections.
CAUTION: It is crucial to exercise caution while employing a cutting instrument, such as a cryotome. To ensure personal safety, adhere to the manufacturer's safety guidelines and keep fingers away from the blade. - Attach a piece of one-sided adhesive tape to the sample by applying firm pressure with the thumb. Beforehand, ensure that the thumb has been cooled by holding it against the chamber wall of the cryotome for a few seconds.
NOTE: The tape should be firmly applied to the sample, as any lack of adhesion may result in the detachment of the tape from the tissue during sectioning. - Produce a 15 µm tissue section with the cryotome. Employ a brush to direct the section and to prevent the adhesive tape from detaching from the sample during the cutting procedure. It is essential for a smooth surface of the tissue section that the cutting pace remains slow and consistent.
- The section created in the previous step now lies flat on the blade block with the tape facing upwards. Use one of the chilled microscope slides with the double-sided tape from the slide box, and firmly press it onto the section to pick it up. Then, place the microscope slide, now carrying the section, back into the slide box.
NOTE: When handling the microscope slide and the section, make sure to keep them within the cryotome chamber at all times to prevent the section from thawing during the cutting procedure. - Repeat the previous three steps until the requisite number of sections, i.e., 40, has been gathered.
- When finished, put the lid on the slide box and transfer it on dry ice into a -20 °C freezer. Finally, clean up the cryotome by disinfecting all used items and turning on the UV light in the cryotome chamber.
NOTE: The protocol can be paused at this point. Store the samples at a temperature of -20 °C until the AFM measurements are performed. The recommended storage time for the sections and OCT medium should not exceed 6 weeks. Beyond this timeframe, the sections and OCT medium may exhibit alterations resembling freezer burn as a result of their substantial surface area and propensity for absorbing moisture.
3. AFM force maps of a lung section
NOTE: For this protocol, the JPK NanoWizard 4XP (AFM) and the motorized stage from Bruker combined with the DMi8 (inverted optical microscope) from Leica was used to record force-displacement curves of the lung sections.
- Setting up the AFM
- Switch on all required devices of the AFM setup (e.g., computer, AFM controller, vibration insulation table, optical microscope, etc.).
- Launch the AFM control software. In the Choose Experiment overview, select the QI Advanced Imaging mode. Additionally, specify the location where the experiment data and calibration files should be stored.
NOTE: If you do not possess the QI Advanced Imaging extension, utilize the Contact Mode Force Mapping mode as an alternate option. - Select a cantilever with a suitable spring constant for the specific type of sample being observed. For the soft lung tissue samples, the MLCT Cantilever F from Bruker with a pyramidal tip and a nominal spring constant of 0.6 N/m is recommended. If a chip contains multiple cantilevers (such as the MLCT), remove any longer cantilevers from the chip using a pair of tweezers under a stereo microscope to ensure that only the cantilever being used for the measurement comes into contact with the sample.
NOTE: Other cantilevers in contact with the sample than the one used for the measurement can lead to undesired movement of the sample during the experiment or even to a detachment of the sample from the underlying tape.
CAUTION: Avoid coming into contact with the cantilever utilized for measurement with the tweezers in the current or subsequent step. - Undertake the installation of the chip onto the cantilever holder. Insert the holder into the AFM head. Position the AFM head on the sample stage, which is situated on the inverted optical microscope. Align the optical lever path as instructed in the user manual. The proper alignment of the optical lever path is achieved when the laser beam is initially placed at the front end of the measurement cantilever, and subsequently, the reflected laser beam hits the center of the segmented photodiode.
- Cantilever calibration using the thermal noise method57
NOTE: The calibration of the cantilever includes determining its inverted optical lever sensitivity (InvOLS) and spring constant. The individual calibration of every used cantilever is necessary because the nominal spring constant typically provided for cantilevers is usually only a rough approximation. In experiments where the mechanical properties of a sample are determined from recorded force curves, such as the one described in this protocol, it is essential to directly determine the InvOLS through a contact-based method on a rigid substrate. Alternative methods determining the InvOLS, such as deriving it from the geometry of the cantilever, can provide only a rough approximation and are not suitable for the type of application described here. Alternatively, if cantilevers are used where the supplier provides the exact spring constant, the InvOLS can be determined by a procedure described by Schiller et al.58.- Place a microscope slide on the sample stage of the AFM. The microscope slide serves as the rigid substrate for the contact-based determination of the InvOLS.
- After placing the AFM head on the sample stage, lower the head using the stepper motors until the remaining gap between the cantilever holder and the microscope slide is only approximately 1 - 2 mm. Apply PBS to the side of the cantilever holder, allowing it to flow down and form a fluid meniscus in the gap between the holder and the microscope slide using a 1 mL syringe with a long needle (0.9 mm x 70 mm).
NOTE: Ensure that the same liquid, such as buffer or media, which is utilized for the force volume measurements of the sample is also employed for the calibration process. - To perform a contact-based calibration in the software, navigate to the Acquire Data page and select the Advanced View option. Access the Calibration Manager by using the burger menu button located in the top right corner. Within this interface, select Contact-based as the Method and choose the MLCT F cantilever from the drop-down menu as the cantilever name. Additionally, set the Setpoint to 1 V and the Number of scans to 10 in the corresponding input fields.
- On the Acquire Data page, in the left-hand control panel, input a setpoint of 1 V for the automatic approach procedure. Click the Blue Downwards Pointing Arrow button located in the top left corner of the user interface to start the automatic approach. When the approach has finished and the cantilever is in contact with the microscope slide, click on the Calibrate button in the Calibration Manager window..
NOTE: The InvOLS and cantilever spring constant are then both determined automatically by recording 10 force curves as well as the thermal power spectral density (PSD), which is used to determine the spring constant with the thermal noise method57. After the automatic calibration procedure is finished, check visually that the fitted curve properly describes the normal mode (first peak) of the recorded PSD of the cantilever and that the determined spring constant has the same order of magnitude as the nominal spring constant given by the cantilever manufacturer. If this is not the case, the fitting range must be manually adjusted to ensure that the fit converges to and represents the first peak of the thermal power spectrum. - After the calibration has finished, close the Calibration Manager window. After closing the Calibration Manager, a calibration file (*.tnd ascii file) in the preselected directory (see step 3.1.2) is generated containing the determined calibration results, which are later required for the data analysis. Document the calibration values for future reference during the data analysis process, for example, in the laboratory notebook.
CAUTION: From this step on, refrain from changing the laser position on the cantilever, adjusting the mirror in the AFM head, or relocating the cantilever holder. Modifying these elements will impact the InvOLS and render the calibration values invalid. To compensate for thermal drift, adjust the position of the segmented photodiode of the AFM head.
- Analyzing an alveolar wall on a lung section
- Retrieve the specimen slide to evaluate from the freezer. Allow the sample to thaw at room temperature for about 1 min until the entire OCT medium has become clear. Subsequently, add some droplets of PBS to the lung tissue section to rehydrate it.
- Place the microscope slide carrying the lung tissue section on the sample stage. Install the AFM head back on the sample stage and lower the head until the cantilever is positioned approximately 1 mm above the sample. Add some additional PBS to the side of the cantilever holder using a 1 mL syringe combined with a 0.9 mm x 70 mm needle until a liquid meniscus between the tissue section and the cantilever holder is formed.
- Navigate to the Settings Manager by clicking on the Button With the Wrench Icon in the upper right corner of the user interface. In the Settings Manager, adjust the following general settings. Within the Approach Settings section, set the Target Height to 4 µm. Then, within the Current Mode Settings section, proceed to the Advanced Feedback Settings and set the Multiplier to 1. Lastly, within the Force Settings subsection, which is also located within the Current Mode Settings section, select the option Retracted Piezo from the Mode at end drop-down menu.
- Use the inverted optical microscope to identify and navigate to an intact alveolar wall. An intact wall is characterized in the bright field image by dark and smooth edges. (see Figure 2A-B).
NOTE: Importing the bright-field image in the AFM control software, when available, can aid in navigating the cantilever to a particular alveolar wall and accurately capturing a force or QI map on top of it. In the JPK SPM software, this function is referred to as Direct Overlay. - Set the parameters for the force-indentation curves in the left-hand control panel based on the AFM used, the cantilever employed, and the observed sample. For the NanoWizard 4XP and the MLCT Cantilever F from Bruker, we recommend the following parameters: Setpoint = 5 nN; z length = 8 µm; z velocity = 300 µm/s.
NOTE: The recommended z length of 8 µm is rather large. This is attributed to the characteristics of lung tissue. Given that lung tissue is typically quite soft, the depth of indentation can reach up to 4 µm at certain points, even with a relatively modest setpoint of 5 nN. In order to guarantee that the baseline, where the tip is not in contact with the sample, accounts for at least about half of the force-indentation curve, it is recommended that the total length of the curve is set to 8 µm. This will significantly simplify the curve analysis later. For other bio-AFMs, these parameters should be applicable as well, although the nomenclature for certain parameters, such as the setpoint, which determines the maximum indentation force, may vary among different manufacturers. If the AFM system is unable to achieve the high vertical velocity given here, it is necessary to decrease the z speed according to the recommendation of your AFM manufacturer.
CAUTION: In order to ensure comparability of the results, it is essential to employ identical z-approach velocities across all experiments. - Set the location and size of the initial overview force map, ensuring that it encompasses the entire alveolar wall, extending from one air side to the other. For murine lung tissue samples, a size between 20 µm x 20 µm and 30 µm x 30 µm is usually sufficient. Regardless of the specific size of the map, set the number of pixels (which correspond to the number of force curves recorded) to 50 x 50. This leads to the generation of 2,500 force-indentation curves for each map, which provides the necessary resolution to identify the BM in the slope channel of the force map. (see Figure 2C-D).
- After recording the overview map, examine the height and slope channel of the force map. If the height channel indicates that the alveolar wall is intact (see Figure 2D) and if it is possible to distinguish the BM in the slope channel as a clearly defined bright line (see Figure 2C,E) situated between two layers of the epithelium and endothelium, capture a more focused force map with a size of 3 µm x 3 µm or 4 µm x 4 µm on the BM. Keep the pixels at 50 x 50 curves.
NOTE: This procedure leads to a higher map resolution and allows recording a greater number of Young's modulus values, particularly for the BM (see Figure 2E-F). Furthermore, this enables a more accurate differentiation between the BM and the surrounding tissues, enhancing the precision of the subsequent spatial filtering process. If the channels of the overview map show a collapsed or ruptured wall, artifacts due to the cantilever touching the sample not only with the tip, or no identifiable BM in the slope channel, navigate to another wall on the tissue section until a wall with an artifact-free assessment of the BM can be identified.
Repeat the previous steps (3.3.6 and 3.3.7) on further sections of the same lung specimen until the desired number of alveolar walls has been assessed. At least four walls are recommended for each lung specimen to achieve robust results59. - Once the measurement is complete, clean all equipment and materials that came into contact with the sample and PBS. If the sample is no longer needed, dispose of it in an autoclavable glass waste container. Save all recorded data, and shut down the AFM control software and computer. Lastly, shut off any electrical devices that were utilized.
NOTE: At this point, the protocol can be paused. The experimental data are saved and can be analyzed at a later time point.
4. Data analysis
- Analysis of force-indentation curves
NOTE: A detailed manual of the Force Curve Analysis application can be found here: https://github.com/CANTERhm/CANTER_Processing_Tool/wiki/2a.-Force-Curve-Analysis.- Launch the CANTER Processing Toolbox56 in MATLAB and open the Force Curve Analysis application.
NOTE: The Toolbox can be downloaded from the GitHub repository by following this link: https://github.com/CANTERhm/CANTER_Processing_Tool. The README.md file, comprising a comprehensive guide on how to install the software and initiate it using MATLAB, is available in the GitHub repository. Further information on each application included in the Toolbox can be found in the corresponding wiki at https://github.com/CANTERhm/CANTER_Processing_Tool/wiki. - Load the high-resolution (3 µm x 3 µm or 4 µm x 4 µm) force map by clicking the Select File button, navigating to the location where the force map is saved, double-clicking on it, and then clicking the Load Data button on the user interface of the application.
- A pop-up window appears, requesting the calibration values (InvOLS and spring constant) that were determined (see step 3.2) for the cantilever used to record the force map selected in the previous protocol step. Enter the calibration values in the respective edit fields. If they have not been documented during step 3.2.4, refer to the automatically generated calibration file, which also contains the calibration values. Keep the default option yes for the question of whether tip-sample separation should be calculated. Subsequently, click the Submit button to proceed.
- A second pop-up window appears, allowing to select the indenter geometry and Poisson ratio for the indented sample. For the used MLCT cantilevers, select Four-sided pyramid as tip geometry and enter 17.5° for the half-angle to the edge (both are the default values). Use the default Poisson ratio of 0.5, which represents an incompressible material. To continue with the loading procedure of the QI map, click on the Submit button.
NOTE: During the loading procedure, the recorded deflection-displacement curves (raw data of the AFM) are converted into force-indentation curves based on the provided calibration values. For a comprehensive understanding of the individual transformation steps and the associated equations, refer to the supplementary information of Hartmann et al.59. - Upon completion of the loading process, the initial force curve of the force map will be displayed on the screen. Ensure that the baseline correction mode Offset + Tilt is selected. This mode automatically detects the baseline of the force-induction curve and corrects the tilt and vertical offset to be zero. Additionally, select via Hertz model as the algorithm for the Contact Point Finder and use a value of 20% in the designated edit field for this option. This algorithm is particularly well-suited for detecting the contact point in force-indentation curves that have been recorded on soft tissue samples. Lastly, set the fit depth in the corresponding edit field to 1.5 µm.
NOTE: To ensure that the rigidity of the microscope slide does not impact the resulting Young's modulus, it is recommended to set the maximum fit depth to a value that is not greater than 10% of the tissue section thickness60. For more details about the correction algorithms, see the supplementary information of Hartmann et al.59.
CAUTION: Use the same fit depth throughout the experiments to guarantee that the results are comparable. - To apply the fit of the modified Hertz model to all force-indentation curves of the QI map, click on the Keep & Apply to all button. A dialog window opens asking whether or not each force curve ought to be displayed during the analysis. By not displaying the curves, the processing speed is slightly accelerated.
- Following the completion of the last force curve analysis, a window appears, asking whether the fit results should be saved. To save the results in both. tsv (tab-separated values) and .xlsx (Excel) file formats, click on Yes and enter a name for the result files. In addition, a text file with the file extension *.meta_data is saved, which contains all the details regarding the analysis of the curves, such as the utilized calibration values, chosen fit model, selected contact point detection algorithm, fit range, and other relevant information.
- Close the Force Curve Analysis application by clicking on the Cross Icon in the top right corner of the main window.
- Launch the CANTER Processing Toolbox56 in MATLAB and open the Force Curve Analysis application.
- Spatial filtering of QI map results
NOTE: The spatial filtering step ensures that only Young's modulus values of the QI maps originating from the BM are taken into account for any further analysis. A detailed manual for the Result Filtering Tool can be found here: https://github.com/CANTERhm/CANTER_Processing_Tool/wiki/Result-Filtering-Tool.- Select the Result Filtering Tool from the list of applications in the application selection window of the CANTER Processing Toolbox and start the application by clicking on the Start Application button.
- To load the fit results, click on Open in the top menu bar of the Result Filtering Tool user interface. In the subsequent pop-up window, locate the first Set button situated within the JPK Maps tab and choose the .tsv file that contains the outcomes of the force curve analysis (refer to step 4.1.6). Click on the second Set button and locate the QI map file that corresponds to the already selected .tsv file. Subsequently, click Submit to load the map data and force curve analysis results.
- Select the Emodul option from the top Displayed channel drop-down menu to display the obtained Young's modulus results as a map image. Furthermore, choose the Emodul option (if not already selected) from the Data channel drop-down menu located beneath the histogram plot to visualize the distribution of the loaded Young's modulus values of the QI map.
- Click on the Manipulation flow arrow button, which is situated in the center of the user interface, and ensure that it points towards the right-hand side. Set the image filter toggle button to the On position in the filter panel located above the histogram axes. Select the Freehand option from the Filter geometry drop-down menu and then click on the Add button.
- In the top channel image, draw the filter mask by circling the BM while holding the left mouse key. The BM is identifiable in the Young's modulus map as a bright (high Young's modulus values) structure compared to the neighboring softer tissue (see Figure 3A). When finished drawing the mask, release the left mouse key and double-click on the mask to be applied to the map. The histogram graph on the right now only shows Young's modulus values for the map regions that are highlighted in green (the default color; see Figure 3C-D).
- To create a new .tsv result file only containing the masked Young's modulus values click in the top menu bar of the user interface on Save > Save histogram > Save data. Alternatively, right-click on an empty area of the histogram plotting axes and select Save data from the context menu.
- Click on Select All in the pop-up window asking which results to write to the .tsv file. Then, confirm the selection by clicking the OK button. Afterward, enter a name for the .tsv file in the save dialog, choose the desired save location, and finally click OK to save the filtered results.
- Close the Result Filtering Tool by clicking on the Cross Icon in the top right corner of the application.
- R2 filtering of results
NOTE: This protocol step ensures that only Young's modulus results are considered where the modified Hertz model showed a good agreement to the force-indentation curve it was fitted to. A detailed manual for the R² Filtering Tool can be found here: https://github.com/CANTERhm/CANTER_Processing_Tool/wiki/R%C2%B2-Filtering-Tool.- Start the R² Filtering Tool by selecting it in the list of applications of the application selection window of the CANTER Processing Toolbox and clicking on Start Application.
- Navigate to the top menu bar of the user interface and click on Open .tsv file. In the subsequent load file dialog, locate the .tsv file containing the Young's modulus results of the BM saved during the previous step 4.2.5, and select it by double-clicking.
- A dialog box appears presenting a list of the data columns contained within the .tsv file. Select Emodul as the column containing the Young's modulus data. Subsequently, in a separate list box dialog requesting the R² data, choose rsquare_fit. Once the loading procedure has been completed, the loaded Young's modulus data is displayed in the form of a histogram on the top left side of the screen, while the R² data distribution is depicted in the graph on the top right side.
- Enter 0.96 in the edit field located in the center of the graphical user interface. Click on the Filter Data button to apply the R² filter by using the entered value. This action will retain only the results that have an R² value of 0.96 or higher. The filtered distributions of the Young's modulus results and the corresponding R² values are displayed in the lower half of the user interface.
- Select Save filtered data from the top menu bar to create a new .tsv file that only includes the results that meet the defined R² filter criterion of > 0.96. The saved data can now be displayed as a histogram, for instance, using the Histogram Plotting Tool, which visualizes Young's modulus distribution for the probed pulmonary BM (see Figure 4A).
Representative Results
After the identification and measurement of an intact alveolar wall, the primary outcome of this protocol is the filtered Young's modulus values of the BM. It is important to note that it is not possible to find a suitable alveolar wall for AFM measurement in every tissue section. In our experimental observations, approximately 75% of the cryosections derived from murine lung specimens exhibited a quantifiable alveolar wall. To accurately determine the spatial distribution of Young's modulus in a pulmonary BM, we advise to employ force maps spanning either 3 µm x 3 µm or 4 µm x 4 µm. This approach ensures sufficient resolution for examining mouse pulmonary BMs, which typically measure 100 nm - 200 nm in thickness. Therefore, the experimental approach involves capturing 50 x 50 force curves for each force map, yielding a total of 2,500 force curves. Within the force map, the force curves are evenly spaced. This implies that in addition to the Young's modulus of the BM, the tissue structures surrounding the BM are probed even in the small force maps. Therefore, to accurately and selectively determine the Young's modulus exclusively of the pulmonary BM, we implemented a two-step filtering process in this protocol.
The initial filtering step of operator-dependent spatial filtering selects force curve fit results originating from the murine pulmonary BM, comprising approximately 10% to 25% of the total result values contained in the analyzed force map. After this initial spatial filtering, approximately 250 - 625 fit results are retained for further analysis. The next filtering step ensures that only the BM-related results originating from force curves adequately described by the modified Hertz model are included. Therefore, the coefficient of determination (R2) of the modified Hertz model fit is used as the filter criterion. Our empirical experience suggests that keeping results with R2 values higher than 0.96 is suitable for fit results obtained by adhering to this protocol. It is important to note that the R2 value is significantly influenced by the quality (smoothness) and length of the force curve's baseline, the determination of the contact point, and the convergence of the fitted model. Following the application of both filtering procedures, approximately 100 to 500 Young's modulus values typically remain for the BM of a single alveolar wall.
The distribution of these remaining Young's modulus values from the BM can be visualized using a histogram (Figure 4A). It is important to note that the resulting Young's modulus values of the pulmonary BM follow a log-normal distribution, which is commonly observed for random variables that can only attain positive values61. This is visualized by the QQ plot in Figure 4B. Consequently, a normal (Gaussian) distribution can be fitted to the distribution of the log-transformed Young's modulus (E) values. Here, the natural logarithm (ln) was used to transform the E values. The peak position of the distribution (µ) and standard deviation (σ) were extracted from this fit and subsequently retransformed to obtain the representative Young's modulus, denoted as EBM, using the following equation:
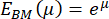
Note that following the retransformation, the standard deviation interval is no longer symmetrical around the peak value. The negative (σ-) and positive (σ+) edges of the standard deviation interval can be calculated using the following expressions:
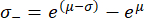
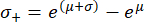
These equations are a consequence of the fact that the exponential function is the inverse function of the natural logarithm62.
After retransforming the peak value μ = 9.31 and the standard deviation σ = 0.18 determined from the histogram in Figure 4A, the representative Young's modulus value is EBM = 11.05 kPa with a standard deviation interval of [-1.82 kPa, +2.18 kPa]. Alternatively, a log-normal distribution can be fitted to the histogram of untransformed Young's modulus (E) values to determine the characteristic parameters of the distribution.
It is recommended that at least four walls per mouse are analyzed to obtain a robust representative result as we have shown in detail in Hartmann et al.59. To determine the representative Young's modulus EBM for a mouse, the log-transformed values of all four walls were plotted in one combined histogram and µ and σ were determined by fitting a normal distribution. Subsequently, µ and σ are retransformed as previously described to retrieve EBM and the standard deviation interval for the subject mouse.
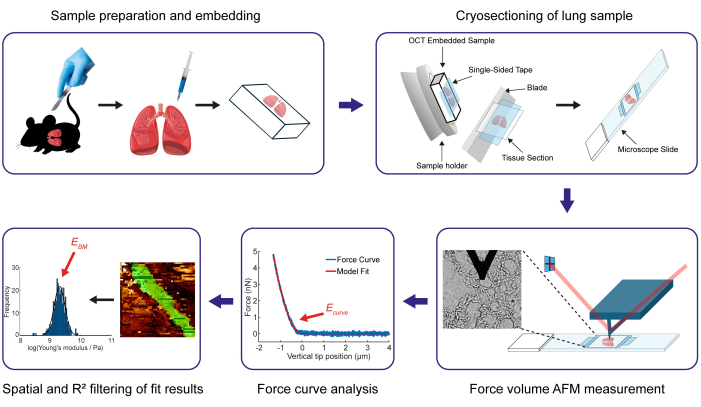
Figure 1: Overview of the main protocol steps to determine the Young's modulus of a murine pulmonary basement membrane. The initial step involves obtaining and preparing murine lungs, followed by embedding them. Hence, the lung is injected with a combination of OCT medium and PBS, and then completely immersed in the OCT compound. Afterwards, 15 µm thick lung tissue sections are obtained through cryosectioning with a cryotome. In this protocol, we detail the process of employing one-sided and two-sided adhesive tapes to secure samples in place during the cutting process. Subsequently, the Young's modulus values of the BM are determined through AFM force map measurements on the tissue sections. Following the completion of the force curve analysis process, spatial and R² filtering is applied to isolate the Young's modulus results of the structure of interest, specifically the BM (shown in green in the force map and quantified in the log-transformed Young's modulus distribution). Please click here to view a larger version of this figure.
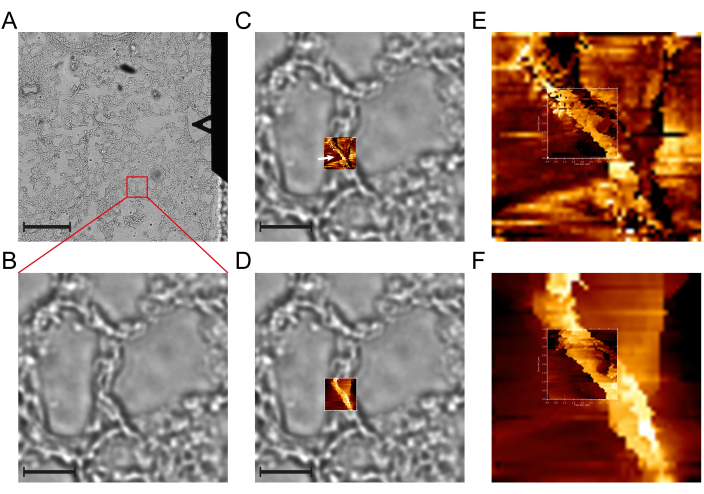
Figure 2: An exemplary force map AFM experiment conducted on a murine lung tissue section. (A) Bright-field microscopy overview image of a murine lung tissue section, enabling the identification of suitable intact alveolar walls situated between two alveoli. On the right side of the image, the triangular-shaped MLCT cantilever F of the AFM is visible. (B) Magnified view of a selected wall within the tissue section. (C) Slope and (D) height channel of an overview 15 x 15 µm map containing 50 x 50 force curves of the alveolar wall. In the slope channel, the BM can be identified as a bright line (white arrow), indicating a structure of increased stiffness within the wall. The height channel confirms the integrity of the wall, showing no evidence of rupture. Following the identification of the BM in the overview 15 x 15 µm map, a smaller 4 x 4 µm map (E: slope channel, F: height channel) with 50 x 50 force curves is recorded. Scale bars: (A) 200 µm and (B-D) 25 µm. The ranges of the color scales of the shown AFM images are (C) 2.36 - 9.29 nN/µm, (D) 0 - 3.38 µm, (E) 2.36 - 9.29 nN/µm (overview image) and 2.42 - 8.37 nN/µm (smaller image), (F) 0 - 3.38 µm (overview image) and 0 - 1.99 µm (smaller image), from dark to bright. Linear color scales were used for all AFM images. Please click here to view a larger version of this figure.
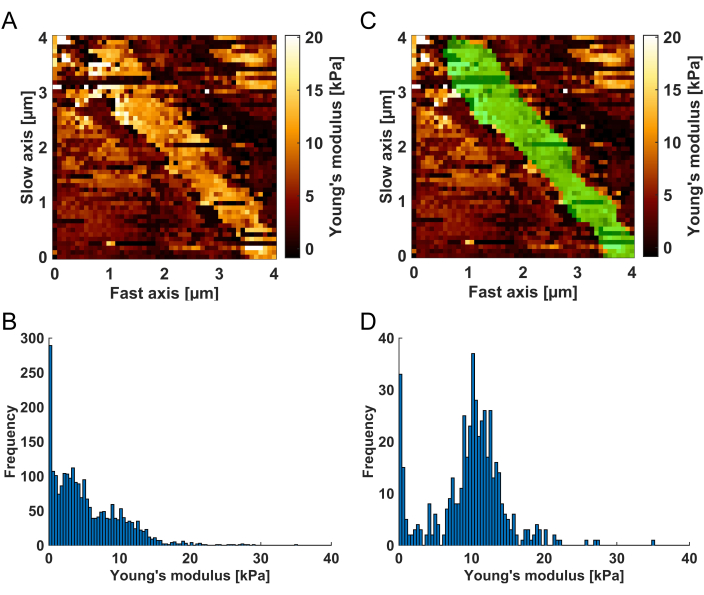
Figure 3: Visualization of the spatial filtering procedure. (A) The Young's modulus map resulting from the force curve analysis, as it is shown in the Result Filtering Tool to enable the spatial filtering of the results. The BM is identifiable as a bright (high Young's modulus values) stripe surrounded by softer tissue compartments. (B) Histogram of the Young's modulus values corresponding to the unfiltered map (A). After the (C) application of the spatial filter mask (green area), (D) the histogram exclusively comprises Young's modulus values from the green highlighted region, which was identified as the BM. Note that the residual soft values observable on the left side of the histogram can be attributed to poorly converging fits, which will be eliminated in the subsequent R² filtering step. Please click here to view a larger version of this figure.
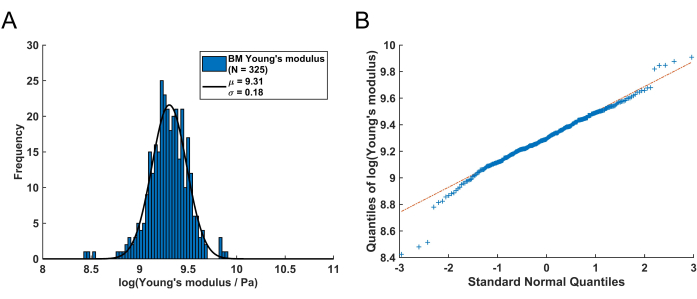
Figure 4: Young's modulus distribution of a murine pulmonary BM received by following this protocol. (A) Log-normalized histogram of the resulting BM Young's modulus values (N = 325). By fitting a normal distribution (black solid line), the mean value µ = 9.31 with a standard deviation of σ = 0.18 is determined. Following retransformation, this corresponds to a Young's modulus of the BM of EBM = 11.05 kPa with an asymmetric standard deviation interval of [-1.82 kPa, +2.18 kPa]. (B) QQ Plot of the quantiles of the logarithmized BM Young's modulus values versus the quantiles of a standard normal distribution. This plot demonstrates that, with the exception of some values in the left and right tails of the distribution, the resulting Young's modulus values are mainly log-normal distributed. Please click here to view a larger version of this figure.
Discussion
The mechanical characteristics of BMs have received considerable interest due to their influence on various cellular functions, such as tumor budding, cellular differentiation, cell movement, and the accessibility of chromatin53,63,64,65,66,67,68. Consequently, there exists an unmet gap in the ability to assess BM mechanics in a consistent and measurable way. This protocol aims to enable AFM users to determine the Young's modulus of pulmonary BMs from healthy or pathological murine lung samples obtained from wildtype or genetically modified mice. The user-friendly graphical user interface based MATLAB application CANTER Processing Toolbox56 facilitates the downstream analysis of AFM data. This protocol is expected to be useful for other researchers conducting studies that could lead to advancements in clinical diagnosis, management, or new treatment strategies for lung disorders.
Critical steps in the protocol include ensuring that during the cryosectioning of the lung specimen, the tissue sections do not thaw at any time to prevent alterations of the mechanical properties due to freeze-thaw cycles. Therefore, it is essential to use only pre-chilled microscope slides to collect the sections and to handle them only within the chamber of the cryotome. The most crucial step during the recording of force maps on an alveolar wall is to utilize the generated slope image, which provides a qualitative overview of the stiffness distribution of the probed area, to identify an appropriate measurement location of the BM. The spatial filtering of the obtained Young's modulus values constitutes a critical step in the protocol and is a key feature of the CANTER Processing Toolbox. This filtering step enables the researcher to identify the BM, which is situated between two softer cell layers, through its distinctive stiffer footprint and extract the corresponding Young's modulus values of the BM for further analysis.
This advanced analysis workflow revealed that netrin-4 knockout mice exhibited a substantially (about two-fold) higher Young's modulus in their pulmonary basement membrane compared to wildtype litter mice53. Thus, the present protocol facilitates the comparative analysis of diverse conditions or groups.
In addition to AFM, numerous methodologies exist for examining the mechanical properties of biomaterials, encompassing optical or magnetic tweezers69,70,71 at the molecular level, micro-indentation techniques72,73 at an intermediate scale and confined or unconfined compression testing74,75 at the macroscopic level. Specifically, pressure myography, tensile testing76,77 and rheology78 have been employed to assess the BM stiffness of vascular samples and reconstituted BM matrices. However, techniques like pressure myography and tensile testing can only assess the mechanical characteristics of complete tissues or whole tissue structures, such as entire blood vessels, which include various cell layers and structural elements. As a result, when using these methods to evaluate BM biomechanics, the mechanical properties of non-BM, such as cellular components, are always superimposed on the BM results, making it difficult to isolate the specific properties of the BM itself.
In this protocol, the feature of interest (BM) is extracted through spatial filtering of the force-map results. A limitation of this approach is the requirement that the feature of interest is discernible and identifiable as a distinct pattern in the obtained Young's modulus maps or any other available map channel. For instance, the BM in alveolar tissue is identifiable solely due to its interconnected structure, which exhibits a significantly higher Young's modulus compared to the surrounding cells. Consequently, the BM manifests as a continuous bright stripe in the obtained Young's modulus maps. Nevertheless, other biological structures exhibit mechanical properties that are not sufficiently distinct from those of the surrounding tissue to enable spatial filtering in the map results. Although the protocol can be combined with other methods to identify structures in the recorded force maps, such as immunofluorescence staining, this approach may yield results that are challenging to interpret. This complication may occur because staining procedures can alter the mechanical properties of tissues. Consequently, any processing or staining of the tissue prior to AFM measurements should be conducted only when no alternative method is available to identify the feature of interest. Moreover, it is important to perform appropriate control experiments comparing the biomechanical properties of stained and unstained samples.
The protocol introduces a method for identifying target structures based on Young's modulus contrast. Consequently, it may be applied to tissues with an anatomical BM similar to that of the lung, including the thyroid gland, colon, and prostate. (see Extended Data Figure in Hartmann et al.59). While initially developed for analyzing BM mechanics, this protocol and the CANTER Processing Toolbox56 are versatile enough to examine any mechanically distinct and connected region within AFM force maps (refer to instructions at www.github.com/CANTERhm/CANTER_Processing_Tool/wiki). This broad applicability makes this tool valuable across the AFM field and supports the increasing focus on mechanobiology in the wider scientific community.
Disclosures
The authors declare no competing interests.
Acknowledgements
B.H., and H.C.-S. acknowledge funding from the Bavarian State Ministry of Science and the Arts through the Bavarian Research Focus Herstellung und biophysikalische Charakterisierung von dreidimensionalen Geweben (CANTER) and the Bavarian Academic Forum (BayWISS)-Doctoral Consortium Health Research. The development of the data analysis software CANTER Processing Toolbox was funded by the German Research Foundation as part of subproject 1 (CL 409/4-1/2) of the research consortium Exploring articular cartilage and subchondral bone degeneration and regeneration in osteoarthritis - ExCarBon (FOR2407-1/2). B.H. and H.C.-S. acknowledge funding from the German Research Foundation through the major instrumentation campaign GGA-HAW (INST 99/38-1). This work was further supported by the Danish Cancer Society (R204-A12454 (R.R.)) and the German Research Foundation (539446614 to R.R.).
Materials
| Name | Company | Catalog Number | Comments |
| 1 mL Syringe | B Braun | 9166017V | Injekt-F |
| 10 mL Syringe | B Braun | 4606108V | Injekt Luer Solo |
| 15 mL Falcon tube | Sarstedt | 62.554.002 | screw cap tube |
| Cantilever - MLCT | Bruker AFM Probes | 3444 | AFM cantilever with a pyramidal tip shape |
| Cryotome blades | Leica Biosystems | 14035843496 | Low-profile disposable blades DB80LX |
| Cryotome sample holder | Leica Biosystems | 14047740044 | Specimen disc 30 |
| Cyotome | Leica Biosystems | CM1950 | Leica Cryostat |
| Direct Overlay Extension | Bruker | Software extension for the JPK SPM Software which enables to import the optical image of the inverted microscope into the Data Viewer of the SPM software. | |
| Disposable base mold | Science Services | SA62534-15 | Tissue-Tek Cryomold 15x15x5 mm |
| Double-sided tape | tesa Film | 56661-00002 | Photo Film Tape |
| Fixed-Spring Cantilever Holder | Bruker | ||
| Inverted Microscope - Leica DMi8 | Leica Microsystems | ||
| JPK Motorized Stage | Bruker | ||
| JPK NanoWizard 4XP BioScience | Bruker | ||
| JPK SPM Software | Bruker | ||
| K5 CMOS Microscope Kamera | Leica Microsystems | ||
| MATLAB | Mathworks | Version R2024a or higher | |
| MATLAB - Curve Fitting Toolbox | Mathworks | ||
| MATLAB - Image Processing Toolbox | Mathworks | ||
| MATLAB - Signal Processing Toolbox | Mathworks | ||
| MATLAB - Statistics and Machine Learning Toolbox | Mathworks | ||
| Microscope slides | Carl Roth | H869.1 | Plain microscope slides for cantilever calibration |
| Microscope slides - frosted edge | Carl Roth | H870.1 | Microscope slides with frosted edge for cryosectioning |
| Needle ø0.9 mm × 25 mm | B Braun | 4657500 | OCT injection into the lung sample |
| Needle ø0.9 mm × 70 mm | B Braun | 4665791 | Long needle to apply PBS under the AFM |
| OCT compound | Sakura Finetek | 4583 | Tissue-Tek O.C.T. Compound |
| Phosphate Buffered Saline | Bio&Sell | BS.L1825 | PBS solution without Ca2+, Mg2+, 500 mL |
| QI Advanced Imaging Extension | Bruker | Software extension for the JPK SPM Software which provides for each recorded image pixel the whole underlying force curve. | |
| Scalpel | B Braun | 5518083 | Surgical Disposable Scalpel |
| Scissors | Kaut-Bullinger | M681700 | Precise Scissors 13 cm |
| Single-sided tape | tesa Film | 57330-00000 | crystal clear tape, 33 m x 19 mm |
| Slide box | GWL Storing Systems | K50W | Slidebox for 50 slides |
| Stereo Microscope - Stemi DR1663 | Zeiss | ||
| Tweezers - Vomm SS-SA-ESD | Eleshop | ELE002121 |
References
- Alsteens, D., et al. Atomic force microscopy-based characterization and design of biointerfaces. Nat Rev Mater. 2 (5), 17008 (2017).
- Aro, E., et al. Severe extracellular matrix abnormalities and chondrodysplasia in mice lacking collagen prolyl 4-hydroxylase isoenzyme II in combination with a reduced amount of isoenzyme I. J Biol Chem. 290 (27), 16964-16978 (2015).
- Domke, J., Radmacher, M. Measuring the elastic properties of thin polymer films with the atomic force microscope. Langmuir. 14 (12), 3320-3325 (1998).
- Huth, S., Sindt, S., Selhuber-Unkel, C. Automated analysis of soft hydrogel microindentation: Impact of various indentation parameters on the measurement of Young's modulus. PLoS One. 14 (8), e0220281 (2019).
- Krieg, M., et al. Atomic force microscopy-based mechanobiology. Nat Rev Phys. 1 (1), 41-57 (2019).
- Loparic, M., et al. Micro- and nanomechanical analysis of articular cartilage by indentation-type atomic force microscopy: validation with a gel-microfiber composite. Biophys J. 98 (11), 2731-2740 (2010).
- Plodinec, M., et al. The nanomechanical signature of breast cancer. Nat Nanotechnol. 7 (11), 757-765 (2012).
- Prein, C., et al. Structural and mechanical properties of the proliferative zone of the developing murine growth plate cartilage assessed by atomic force microscopy. Matrix Biol. 50, 1-15 (2016).
- Radmacher, M., Fritz, M., Kacher, C. M., Cleveland, J. P., Hansma, P. K. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophys J. 70 (1), 556-567 (1996).
- Radmacher, M., Tillamnn, R. W., Fritz, M., Gaub, H. E. From molecules to cells: imaging soft samples with the atomic force microscope. Science. 257 (5078), 1900-1905 (1992).
- Stolz, M., et al. Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy. Nat Nanotechnol. 4, 186-192 (2009).
- Stolz, M., et al. Dynamic elastic modulus of porcine articular cartilage determined at two different levels of tissue organization by indentation-type atomic force microscopy. Biophys J. 86 (5), 3269-3283 (2004).
- Florin, E. L., Moy, V. T., Gaub, H. E. Adhesion forces between individual ligand-receptor pairs. Science. 264 (5157), 415-417 (1994).
- Hansma, P. K., et al. Tapping mode atomic force microscopy in liquids. Appl Phys Lett. 64 (13), 1738-1740 (1994).
- Moy, V. T., Florin, E. L., Gaub, H. E. Intermolecular forces and energies between ligands and receptors. Science. 266 (5183), 257-259 (1994).
- Radmacher, M., Fritz, M., Hansma, H. G., Hansma, P. K. Direct observation of enzyme activity with the atomic force microscope. Science. 265 (5178), 1577-1579 (1994).
- Radmacher, M., Fritz, M., Hansma, P. K. Imaging soft samples with the atomic force microscope: gelatin in water and propanol. Biophys J. 69 (1), 264-270 (1995).
- Rief, M., Clausen-Schaumann, H., Gaub, H. E. Sequence-dependent mechanics of single DNA molecules. Nat Struct Biol. 6 (4), 346-349 (1999).
- Rief, M., Gautel, M., Oesterhelt, F., Fernandez, J. M., Gaub, H. E. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science. 276 (5315), 1109-1112 (1997).
- Drake, B., et al. Imaging crystals, polymers, and processes in water with the atomic force microscope. Science. 243 (4898), 1586-1589 (1989).
- Clausen-Schaumann, H., Rief, M., Tolksdorf, C., Gaub, H. E. Mechanical stability of single DNA molecules. Biophys J. 78 (4), 1997-2007 (2000).
- Rotsch, C., Radmacher, M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophys J. 78 (1), 520-535 (2000).
- Lekka, M., et al. Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. Eur Biophys J. 28 (4), 312-316 (1999).
- Weisenhorn, A. L., Khorsandi, M., Kasas, S., Gotzos, V., Butt, H. J. Deformation and height anomaly of soft surfaces studied with an AFM. Nanotechnology. 4 (2), 106 (1993).
- Rotsch, C., Jacobson, K., Radmacher, M. Dimensional and mechanical dynamics of active and stable edges in motile fibroblasts investigated by using atomic force microscopy. Proc Natl Acad Sci USA. 96 (3), 921-926 (1999).
- Goldmann, W. H., Ezzell, R. M. Viscoelasticity in wild-type and vinculin-deficient (5.51) mouse F9 embryonic carcinoma cells examined by atomic force microscopy and rheology. Exp Cell Res. 226 (1), 234-237 (1996).
- Tschaikowsky, M., et al. Hybrid fluorescence-AFM explores articular surface degeneration in early osteoarthritis across length scales. Acta Biomater. 126, 315-325 (2021).
- Tschaikowsky, M., et al. Proof-of-concept for the detection of early osteoarthritis pathology by clinically applicable endomicroscopy and quantitative AI-supported optical biopsy. Osteoarthr Cartilage. 29 (2), 269-279 (2021).
- Kinney, J. H., Balooch, M., Marshall, S. J., Marshall, G. W., Weihs, T. P. Atomic force microscope measurements of the hardness and elasticity of peritubular and intertubular human dentin. J Biomech Eng. 118 (1), 133-135 (1996).
- Lundkvist, A., et al. . Symposium on Thin Films - Stresses and Mechanical Properties VI,. 1996 MRS Spring Meeting. , 353-358 (1997).
- Tao, N. J., Lindsay, S. M., Lees, S. Measuring the microelastic properties of biological material. Biophys J. 63 (4), 1165-1169 (1992).
- Radmacher, M. Measuring the elastic properties of biological samples with the AFM. IEEE Eng Med Biol Mag. 16 (2), 47-57 (1997).
- Becke, T. D., et al. Pilus-1 backbone protein RrgB of Streptococcus pneumoniae binds collagen I in a force-dependent way. ACS Nano. 13 (6), 7155-7165 (2019).
- Becke, T. D., et al. Single molecule force spectroscopy reveals two-domain binding mode of Pilus-1 tip protein RrgA of Streptococcus pneumoniae to fibronectin. ACS Nano. 12 (1), 549-558 (2018).
- Pill, M. F., East, A. L. L., Marx, D., Beyer, M. K., Clausen-Schaumann, H. Mechanical activation drastically accelerates amide bond hydrolysis, matching enzyme activity. Angew Chem Int Ed Engl. 58 (29), 9787-9790 (2019).
- Schmidt, S. W., Filippov, P., Kersch, A., Beyer, M. K., Clausen-Schaumann, H. Single-molecule force-clamp experiments reveal kinetics of mechanically activated silyl ester hydrolysis. ACS Nano. 6 (2), 1314-1321 (2012).
- Docheva, D., et al. Researching into the cellular shape, volume and elasticity of mesenchymal stem cells, osteoblasts and osteosarcoma cells by atomic force microscopy. J Cell Mol Med. 12 (2), 537-552 (2008).
- Docheva, D., Padula, D., Schieker, M., Clausen-Schaumann, H. Effect of collagen I and fibronectin on the adhesion, elasticity and cytoskeletal organization of prostate cancer cells. Biochem Biophys Res Commun. 402 (2), 361-366 (2010).
- Kiderlen, S., et al. Age related changes in cell stiffness of tendon stem/progenitor cells and a rejuvenating effect of ROCK-inhibition. Biochem Biophys Res Commun. 509 (3), 839-844 (2019).
- Reuten, R., et al. Structural decoding of netrin-4 reveals a regulatory function towards mature basement membranes. Nat Commun. 7, 13515 (2016).
- Fleischhauer, L., et al. Nano-scale mechanical properties of the articular cartilage zones in a mouse model of post-traumatic osteoarthritis. Appl Sci. 12 (5), 2596 (2022).
- Alberton, P., et al. Aggrecan hypomorphism compromises articular cartilage biomechanical properties and is associated with increased incidence of spontaneous osteoarthritis. Int J Mol Sci. 20 (5), 1-16 (2019).
- Alberton, P., et al. Aggrecan is critical in maintaining the cartilage matrix biomechanics which in turn influences the correct development of the growth plate. Osteoarthr Cartilage. 27, S178-S178 (2019).
- Gronau, T., et al. Forced exercise-induced osteoarthritis is attenuated in mice lacking the small leucine-rich proteoglycan decorin. Ann Rheum Dis. 76 (2), 442-449 (2017).
- Hartmann, B., et al. Early detection of cartilage degeneration: A comparison of histology, fiber Bragg grating-based micro-indentation, and atomic force microscopy-based nano-indentation. Int J Mol Sci. 21 (19), 7384-7398 (2020).
- Rellmann, Y., et al. ER stress in ERp57 knockout knee joint chondrocytes induces osteoarthritic cartilage degradation and osteophyte formation. Int J Mol Sci. 23 (1), 182 (2021).
- Kamper, M., et al. Early changes in morphology, bone mineral density and matrix composition of vertebrae lead to disc degeneration in aged collagen IX -/- mice. Matrix Biol. 49, 132-143 (2016).
- Akhtar, R., Sherratt, M. J., Cruickshank, J. K., Derby, B. Characterizing the elastic properties of tissues. Mater Today. 14 (3), 96-105 (2011).
- Junior, C., et al. Baseline stiffness modulates the non-linear response to stretch of the extracellular matrix in pulmonary fibrosis. Int J Mol Sci. 22 (23), 12928 (2021).
- Junior, C., et al. Multi-step extracellular matrix remodelling and stiffening in the development of idiopathic pulmonary fibrosis. Int J Mol Sci. 24 (2), 1708 (2023).
- Sicard, D., Fredenburgh, L. E., Tschumperlin, D. J. Measured pulmonary arterial tissue stiffness is highly sensitive to AFM indenter dimensions. J Mech Behav Biomed Mater. 74, 118-127 (2017).
- Zemla, J., et al. AFM-based nanomechanical characterization of bronchoscopic samples in asthma patients. J Mol Recognit. 31 (12), e2752 (2018).
- Reuten, R., et al. Basement membrane stiffness determines metastases formation. Nat Mater. 20 (6), 892-903 (2021).
- Johnstone, A. H. CRC Handbook of Chemistry and Physics. J Chem Technol Biotechnol. 50 (2), 294-295 (1991).
- Rico, F., et al. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys Rev E. 72 (2), 1-10 (2005).
- . . CANTER Processing Toolbox v.5.7.0. , (2022).
- Hutter, J. L. J. B. Calibration of atomic-force microscope tips. Rev Sci Instrum. 64 (7), 1868-1873 (1993).
- Schillers, H., et al. Standardized nanomechanical atomic force microscopy procedure (SNAP) for measuring soft and biological samples. Sci Rep. 7 (1), 5117 (2017).
- Hartmann, B., et al. Profiling native pulmonary basement membrane stiffness using atomic force microscopy. Nat Protoc. 19 (5), 1498-1528 (2024).
- Dimitriadis, E. K., Horkay, F., Maresca, J., Kachar, B., Chadwick, R. S. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys J. 82 (5), 2798-2810 (2002).
- Jolicoeur, P. . Introduction to Biometry. , (1999).
- Bronshtein, I. N., Semendyayev, K. A., Musiol, G., Mühlig, H. . Handbook of Mathematics. , (2015).
- Fiore, V. F., et al. Mechanics of a multilayer epithelium instruct tumour architecture and function. Nature. 585 (7825), 433-439 (2020).
- Koester, J., et al. Niche stiffening compromises hair follicle stem cell potential during ageing by reducing bivalent promoter accessibility. Nat Cell Biol. 23 (7), 771-781 (2021).
- Bedzhov, I., Zernicka-Goetz, M. Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell. 156 (5), 1032-1044 (2014).
- Kyprianou, C., et al. Basement membrane remodelling regulates mouse embryogenesis. Nature. 582 (7811), 253-258 (2020).
- Saraswathibhatla, A., Indana, D., Chaudhuri, O. Cell-extracellular matrix mechanotransduction in 3D. Nat Rev Mol Cell Biol. 24 (7), 495-516 (2023).
- Sherwood, D. R. Basement membrane remodeling guides cell migration and cell morphogenesis during development. Curr Opin Cell Biol. 72, 19-27 (2021).
- Ashkin, A., Dziedzic, J. M., Bjorkholm, J. E., Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt Lett. 11 (5), 288 (1986).
- Marago, O. M., Jones, P. H., Gucciardi, P. G., Volpe, G., Ferrari, A. C. Optical trapping and manipulation of nanostructures. Nat Nanotechnol. 8 (11), 807-819 (2013).
- Neuman, K. C., Nagy, A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat Methods. 5 (6), 491-505 (2008).
- Marchi, G., et al. Microindentation sensor system based on an optical fiber Bragg grating for the mechanical characterization of articular cartilage by stress-relaxation. Sens Actuators B. 252, 440-449 (2017).
- Wakitani, S., et al. Repair of large full-thickness articular cartilage defects with allograft articular chondrocytes embedded in a collagen gel. Tissue Eng. 4 (4), 429-444 (1998).
- Moutos, F. T., Freed, L. E., Guilak, F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater. 6 (2), 162-167 (2007).
- Schwarz, S., et al. Contactless vibrational analysis of transparent hydrogel structures using laser-Doppler vibrometry. Exp Mech. 60 (8), 1067-1078 (2020).
- Bhave, G., Colon, S., Ferrell, N. The sulfilimine cross-link of collagen IV contributes to kidney tubular basement membrane stiffness. Am J Physiol Renal Physiol. 313 (3), F596-F602 (2017).
- Fisher, R. F., Wakely, J. The elastic constants and ultrastructural organization of a basement membrane (lens capsule). Proc R Soc Lond B Biol Sci. 193 (1113), 335-358 (1976).
- Wisdom, K. M., et al. Covalent cross-linking of basement membrane-like matrices physically restricts invasive protrusions in breast cancer cells. Matrix Biol. 85-86, 94-111 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved