Bu içeriği görüntülemek için JoVE aboneliği gereklidir. Oturum açın veya ücretsiz deneme sürümünü başlatın.
Retroviral-Mediated Transduction: A Method to Introduce Target Gene into Cancer Cells
Overview
This video describes the protocol for genetic validation by transferring target genes using retroviruses in chronic myeloid leukemia cell lines. The retroviruses act as a mediator to transfer genetic material into the leukemia cell through the process called transduction, which enables long term expression of target gene.This method can be applied to any target cells for genetic validation and therapeutic development.
Protokol
1. Transduction
- Retroviral plasmid transfection
- Freshly thaw HEK293 cells the afternoon before in a water bath set to 37 °C. Spin the cryotube containing the HEK cells at 435 x g at room temperature for 3 min. Remove the supernatant via a vacuum and suspend the cell pellet in 1 mL of DMEM supplemented with 10% FBS, penicillin, streptomycin, and glutamine.
- Count the cells and add the appropriate volume to a 10 cm treated dish (~4 x 106 HEK293T cells). Add 14 mL of the DMEM 10 media to the dish and mix it well by sliding up and down for an equal distribution. Place the dish in a 37 °C, 5% CO2 incubator overnight.
- On the next morning, check the confluence of the cells under an inverted light microscope (50% or more works well).
- Combine the DNA (desired retroviral plasmid), PclEco (packaging plasmid), 2 M CaCl2, and H20 in a 1.5 mL tube (= 500 µL) using a micropipette in the following amounts: 7.5 µg of DNA (BCR-ABL1-YFP), 7.5 µg of pCLeco, 62 µL of 2 M CaCl2, and water to a final volume of 500 µL. Add 500 µL of 2x HBS to another 1.5 mL tube.
- Add the DNA mix dropwise to the 1.5 mL tube containing 500 µL of 2x HBS (280 mM NaCl, 100 mM HEPES, and 1.5 mM Na2HPO4) while mixing. Achieve this by setting a vortex to the lowest speed and hold the HBS tube on it for constant mixing while adding the transfection mix.
- Allow the transfection mix to incubate for 20–30 min at room temperature. During the incubation, add 25 nM chloroquine to the HEK cells and place them back in the incubator. After the incubation, add the transfection mix dropwise to the HEK cells and, then, gently swirl the media to mix the transfection mix throughout the plate.
- Incubate the cells with the mix for ~8 h in a 37 °C, 5% CO2 incubator. Change the media on these cells by very slowly adding 14 mL of fresh DMEM 10 media. Pipetting slowly against the wall of the dish helps to prevent any stripping of the HEK cells. Incubate the cells overnight in the 37 °C, 5% CO2 incubator.
- Collect the viral supernatant with a 10 mL syringe, draw the supernatant into the syringe, and attach the 0.45 µm syringe filter. Plunge the viral supernatant into a new collection tube. Take the first collection of viral supernatant ~16 h later.
- Fibronectin viral concentration and transduction
- Add 2 mL of a 0.1 mg recombinant human fibronectin fragment in 1x PBS to untreated 6-well culture plates. Prepare as much fibronectin as needed, which depends on the number of cells and genotypes. One well can sufficiently transduce 10 million c-Kit+ cells. Incubate the plate overnight at 4 °C.
- The next day, remove the fibronectin from the wells with a pipette, pipette 2 mL of sterile 2% BSA in 1x PBS to block the plate, and incubate for 30 min at room temperature. Remove the BSA via a vacuum and wash thoroughly by pipetting 2 mL of 1x PBS then remove it via a vacuum.
- Immediately add freshly collected and filtered (at 0.45 µm) viral supernatant up to 5 mL. Centrifuge at 435 x g at 32 °C for 2 h. Remove the supernatant via a vacuum and repeat this step with additional freshly collected viral supernatant and spin again. Remove the supernatant via a vacuum and wash the wells by adding 2 mL of 1x PBS; then, remove it via a vacuum.
- Add the c-Kit+ mouse cells that were cultured overnight by spinning down the cells and resuspending the pellet in complete IMDM ,for culture. Culture these cells overnight in 2 x 106 cells/1 mL of cell IMDM complete media by placing them in an incubator at 37 °C and 5% CO2) to the viral coated wells by mixing the cell suspensions well and pipetting them into the now viral concentrated fibronectin plate. Spin at 1,200 x g at 25 °C for 90 min. Put the cells in a 37 °C, 5% CO2 incubator.
- 48 h post transduction, pellet the cells by centrifugation at 1,200 x g at 4 °C for 5 min and resuspend them in FACS buffer #1 (1x PBS + 0.5% BSA) to 6 x 106/mL. Determine the percentage of BCR-ABL1 positive cells by measuring the percentage of YFP positive using flow cytometry.
- Acquire events with the standard procedure. First, gate the live cells based on forward and side scatter. Then, gate the YFP positive live cells excluding the YFP-negative cells determined by negative control (Figure 1).
Sonuçlar
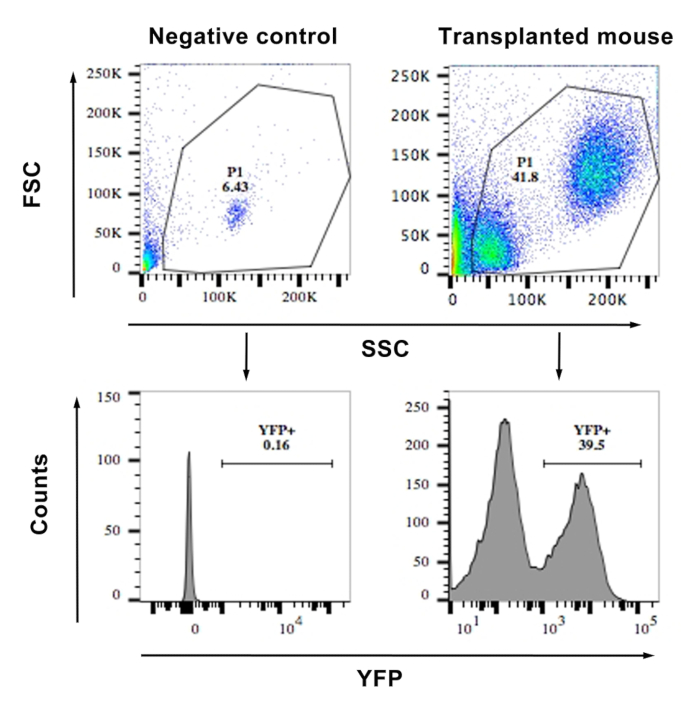
Figure 1: FACS showing the frequency of YFP+ cells from the blood of a transplanted and a WT mouse (negative control). The top panels show a scatter plot of the cells and the gating. The lower panels show histograms of YFP vs. side scatter. Cells with an MFI of >103 were considered YFP+. Similar gating was used for bone marrow cell...
Açıklamalar
Malzemeler
| Name | Company | Catalog Number | Comments |
| DMEM | Cellgro (corning) | 15-013-CV | |
| RetroNectin Recombinant Human Fibronectin Fragment | Takara | T100B | |
| FBS | Atlanta biological | S11150 | |
| Penn/Strep | Cellgro (corning) | 30-002-CI | |
| L-Glutamine | Cellgro (corning) | 25-005-CL | 5 mg/mL stock in water |
| HEPES | Sigma | H7006 | |
| PBS | Corning | 21040CV | |
| Na2HPO4.7H2O | Sigma | S9390 | |
| Calcium Chloride | Invitrogen | K278001 | |
| 2x HBS | Invitrogen | K278002 | |
| EDTA | Ambion | AM9261 | |
| BSA | Sigma | A7906 | |
| Human Long-Term Culture Initiating Cell Assay | Stemp Cell Technologies | ||
| TC-10 automated cell counter | Bio-RAD | ||
| ROSACreERT2/c-Fos fl/fl Dusp1-/- | Made in house | ||
| ROSACreERT2/c-Fosfl/fl | Made in house | ||
| ROSACreERT2 | Jackson Laboratory | ||
| C57Bl/6 | Jackson Laboratory | ||
| CML-CD34+ and Normal CD34+ cells | University Hospital, University of Cincinnati | ||
| LSR II (FACS analyzer) | BD | ||
| Fortessa I (FACS analyzer) | BD | ||
| FACSAriaII (FACS Sorter) | BD | ||
| NAPCO series 8000 WJ CO2 incubator | Thermo scientific | ||
| Swing bucket rotor cetrifuge 5810R | Eppendorf | ||
| 1/2 cc Lo-Dose u-100 insulin syringe 28 G1/2 | Becton Dickinson | Becton Dickinson | |
| Hydrocortisone Sodium Hemisuccinate | Stem Cell Technologies | 7904 | |
| GM-CSF | Prospec | CYT-221 | |
| G-SCF | Prospec | CYT-220 | |
| Human IL-3 | Prospec | CYT-210 | |
| human SCF | Prospec | CYT-255 | |
| Erythropoiein | Amgen | 5513-267-10 | |
| BD Perm/Wash (permeabilization and wash solution for phospho flow) | BD | 554723 | |
| BD Cytofix/Cytoperm (Fixing and permeabilization solution) | BD | 554714 | |
| BD Pharm Lyse | BD | 555899 | |
| 70 μm nylon cell stariner | Becton Dickinson | 352350 | |
| 0.45 μM acro disc filter | PALL | 2016-10 |
This article has been published
Video Coming Soon
Source: Kesarwani, M., et al. Methods for Evaluating the Role of c-Fos and Dusp1 in Oncogene Dependence. J. Vis. Exp. (2019).
JoVE Hakkında
Telif Hakkı © 2020 MyJove Corporation. Tüm hakları saklıdır