Bu içeriği görüntülemek için JoVE aboneliği gereklidir. Oturum açın veya ücretsiz deneme sürümünü başlatın.
Assessing Vessel Perfusion via Angiography: A Technique to Introduce Contrast Agent in Descending Aorta to Visualize Blood Vessel Perfusion in Rabbit Model
Overview
This video describes the technique for performing angiography by injecting a contrast agent into the descending aorta to visualize blood vessel perfusion in a rabbit model. This technique helps to understand the perfusion in blood vessels with blockages and after treatment during recovery.
Protokol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparation of rabbit for surgery
- Anesthetize the rabbit using 20–40 mg/kg ketamine and 2 mg/kg midazolam via subcutaneous injection. Place the rabbit on 1.5%–3% isoflurane (typically 2%) throughout the initial sedation using a mask. Give an injection of alfaxalone to maintain anesthesia via an intramuscular injection of 3 mg/kg.
- Once anesthetized, remove the mask and insert a cuffed endotracheal tube, into the airway and connect to a ventilator. Continue to administer isoflurane at 1.5%–3%.
- Collect blood from the central artery from either ear for a baseline chemistry panel.
- Place a 22 G ear vein catheter in the lateral ear vein for Lactated Ringer's Solution drip throughout the surgical procedure. Alternatively, normal saline (0.9% sodium chloride) can be used.
- Using the lateral vein in the opposite ear, place a catheter in the vein and deliver alfaxalone at 6 mg/kg/h. Gradually increase the alfaxalone to 8 mg/kg/h while decreasing isoflurane to 0.6% during the prep period.
- To limit pain and risk of infection, administer buprenorphine (0.01 mg/kg) and enrofloxacin (5 mg/kg) using a subcutaneous injection with a 25 G needle.
- Trim the hair on the neck, right and left inner thighs, and back using clippers (#40 blade). Hair is removed from the back to maintain contact with the grounding pad.
- Place a blood pressure cuff on each of the hind limbs and measure initial blood pressure. Place the cuff just below the knee with the probe just above the hock on the lateral surface.
- Position the rabbit on the surgery table on its back and scrub and drape the surgery sites. This includes the neck for carotid artery access and inner right thigh for femoral artery access. Perform the sterilization scrub with alternating scrubs of 2% chlorhexidine and 70% ethyl alcohol. Repeat this three times, then apply a final spray with 2% chlorhexidine solution.
- Place a 3-mm stainless steel ball that has been sterilized inside a low density polyethyene bag on top of the right (scrubbed) leg near the upper part of the thigh to serve as a size reference during angiogram measurements. Place a sterile drape over the leg until the time of surgery. Leave the ball inside the sterile plastic bag during the first angiogram.
2. Angiography
- Expose the right common carotid artery
- Make a 4–5 cm long incision just lateral to the trachea using a scalpel with a #15 blade.
- Use blunt dissection to expose the carotid artery and open the incision using small Weitlaner retractors. Carefully isolate the carotid artery from the jugular vein and vagus nerve. Typically, a curved Metzenbaum scissors and a curved mosquito hemostat are used for the blunt dissection. Be sure to get full separation of the carotid artery from the nerve and jugular vein to make the ligatures only ligate the artery.
- Place a ligature using a 4-0 silk suture at the proximal and distal ends of the exposed artery. Tie off the distal end of carotid with a surgeon’s knot followed by four square knots. On the proximal end, use a ligaloop to allow it to be tightened or loosened as needed. The use of a ligaloop placed at the proximal end of the exposed artery can help secure the introducer and catheter.
- Administer 500 IU of heparin through the IV. Use approximately 0.5 mL of 1% lidocaine applied along the exposed carotid to dilate the vessel. One treatment is usually sufficient, but it can be repeated as needed. Cut approximately half way through the carotid artery using a scalpel or iris scissors, then place the 4-inch wire insertion tool into the artery.
- Feed a 0.014 inch x 185 cm guidewire through the insertion tool to the aortic bifurcation at the iliac crest in the descending aorta. Remove the insertion tool and insert a 3F pigtail angiographic catheter over the wire.
- Advance the pigtail catheter to be 2 cm proximal to the aortic bifurcation at the iliac crest in the descending aorta.
- Position the tip of the catheter between the seventh lumbar and first sacral vertebrae. Test the location of the catheter by manually injecting a 2–4 mL of contrast agent.
- Administer an intra-arterial injection of 100 μg nitroglycerin through the catheter to increase vasodilation.
- Administer 0.8 mL of 1% lidocaine to the rabbit through the catheter to assist with vasodilation during the angiogram. Attach the tubing for the injector to the catheter and remove any air bubbles in the line. Inject 8-9 mL of contrast media using automated angiographic injector through the catheter.
- Record serial images of the hind limbs using angiography.
- Set the power injector to inject contrast at 3 mL/sec for a total of 8-9 mL. Perform digital subtraction angiography at 6 frames per second.
- Select the serial images created and alter a photo of each angiogram using approximately -40% setting to minimize appearance of bone and capture a complete picture of the vessel perfusion with contrast. An example angiogram of the vascular flow after femoral artery ligation/excision is shown in Figure 1.
Access restricted. Please log in or start a trial to view this content.
Sonuçlar
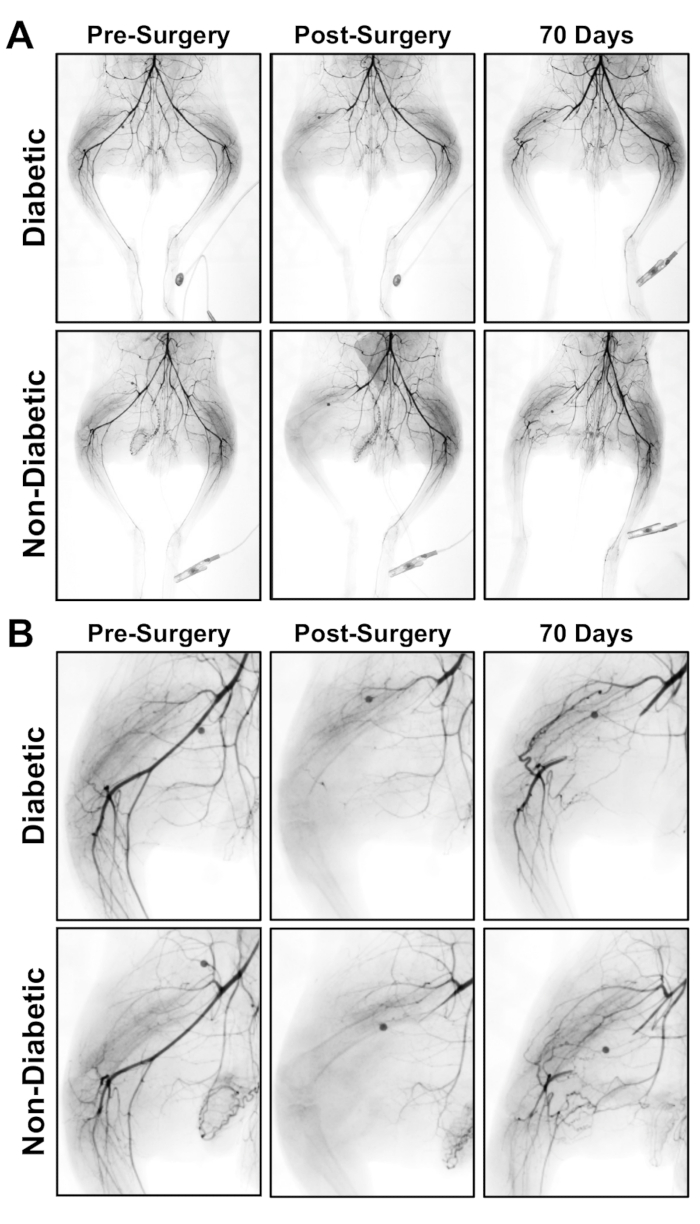
Figure 1: Angiograms for the hind limb of a diabetic and non-diabetic rabbit pre-surgery, post-surgery and after recovery for 70 days after femoral artery ligation and excision. (A) Angiogram of ischemic limb (left) and contralateral control limb (right). (B) Enlarged image of the ischemic limb at the site of ligation.
Access restricted. Please log in or start a trial to view this content.
Açıklamalar
Malzemeler
| Name | Company | Catalog Number | Comments |
| Angiography Equipment | Toshiba | Infinix-i | |
| Guidewire | Boston Scientific | 39122-01 | |
| Insertion Tool | Merit Medical Systems | MAP550 | metal wire insertion tool |
| Lidocaine | McKesson Medical-Surgical | 239936 | |
| Ligaloop | V. Mueller | CH117 / CH116 | White Mini / Yellow Mini |
| Pigtail Catheter | Merit Medical Systems | 1310-21-0053 | 3F pigtail |
| Silk Sutures | Ethicon-Johnson & Johnson | A183H | 4-0 silk ties 18" |
| Syringe Pump | DRE Veterinary | Versaflow VF-300 | |
| Visipaque contrast media | McKesson Medical-Surgical | 509055 | |
| Angiography Injector | Medrad |
Referanslar
Access restricted. Please log in or start a trial to view this content.
This article has been published
Video Coming Soon
Source: Sligar, A. D et al., Preclinical Model of Hind Limb Ischemia in Diabetic Rabbits. J. Vis. Exp. (2019).
JoVE Hakkında
Telif Hakkı © 2020 MyJove Corporation. Tüm hakları saklıdır