Gas Chromatography (GC) with Flame-Ionization Detection
Source: Laboratory of Dr. B. Jill Venton - University of Virginia
Gas chromatography (GC) is used to separate and detect small molecular weight compounds in the gas phase. The sample is either a gas or a liquid that is vaporized in the injection port. Typically, the compounds analyzed are less than 1,000 Da, because it is difficult to vaporize larger compounds. GC is popular for environmental monitoring and industrial applications because it is very reliable and can be run nearly continuously. GC is typically used in applications where small, volatile molecules are detected and with non-aqueous solutions. Liquid chromatography is more popular for measurements in aqueous samples and can be used to study larger molecules, because the molecules do not need to vaporize. GC is favored for nonpolar molecules while LC is more common for separating polar analytes.
The mobile phase for gas chromatography is a carrier gas, typically helium because of its low molecular weight and being chemically inert. Pressure is applied and the mobile phase moves the analyte through the column. The separation is accomplished using a column coated with a stationary phase. Open tubular capillary columns are the most popular columns and have the stationary phase coated on the walls of the capillary. Stationary phases are often derivatives of polydimethylsiloxane, with 5–10% of the groups functionalized to tune the separation. Typical functional groups are phenyl, cyanopropyl, or trifluoropropyl groups. Capillary columns are usually 5–50 m long. Narrower columns have higher resolution but require higher pressures. Packed columns can also be used where the stationary phase is coated onto beads packed in the column. Packed columns are shorter, 1–5 m. Open tubular capillaries are generally preferred because they allow higher efficiencies, faster analyses, and have higher capacities.
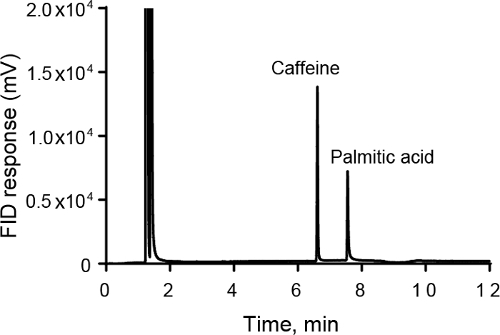
Flame-ionization detection (FID) is a good general detector for organic compounds in GC that detects the amount of carbon in a sample. After the column, samples are burned in a hot, hydrogen-air flame. Carbon ions are produced by the combustion. While the overall efficiency of the process is low (only 1 in 105 carbon ions produce an ion in the flame) the total amount of ions is directly proportional to the amount of carbon in the sample. Electrodes are used to measure the current from the ions. FID is a destructive detector, as the entire sample is pyrolyzed. FID is unaffected by noncombustible gases and water.
1. Initialization of the GC
- Turn on the helium carrier gas and air and adjust the pressure gauges on the instrument.
- Turn on the column oven to a high temperature (typically 250 °C or above) to bake in the column. Do not exceed the maximum temperature of the column. This will remove any contaminants. Let it bake for at least 30 min before running a sample.
2. Making a Methods File
- In the software controlling the instrument, input all the desired va
GC is used for a variety of industrial applications. For example, it is used to test the purity of a synthesized chemical product. GC is also popular in environmental applications. GC is used to detect pesticides, polyaromatic hydrocarbons, and phthalates. Most air quality applications use GC-FID to monitor environmental pollutants. GC is also used for headspace analysis, where the volatiles that are evaporated from a liquid are collected and measured. This is useful for the cosmetic and food and beverage industries. GC
Skip to...
Videos from this collection:

Now Playing
Gas Chromatography (GC) with Flame-Ionization Detection
Analytical Chemistry
279.5K Views

Sample Preparation for Analytical Characterization
Analytical Chemistry
83.5K Views

Internal Standards
Analytical Chemistry
203.1K Views

Method of Standard Addition
Analytical Chemistry
318.5K Views

Calibration Curves
Analytical Chemistry
788.5K Views

Ultraviolet-Visible (UV-Vis) Spectroscopy
Analytical Chemistry
615.8K Views

Raman Spectroscopy for Chemical Analysis
Analytical Chemistry
50.7K Views

X-ray Fluorescence (XRF)
Analytical Chemistry
25.2K Views

High-Performance Liquid Chromatography (HPLC)
Analytical Chemistry
381.3K Views

Ion-Exchange Chromatography
Analytical Chemistry
262.8K Views

Capillary Electrophoresis (CE)
Analytical Chemistry
92.9K Views

Introduction to Mass Spectrometry
Analytical Chemistry
111.3K Views

Scanning Electron Microscopy (SEM)
Analytical Chemistry
86.5K Views

Electrochemical Measurements of Supported Catalysts Using a Potentiostat/Galvanostat
Analytical Chemistry
51.2K Views

Cyclic Voltammetry (CV)
Analytical Chemistry
123.2K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved