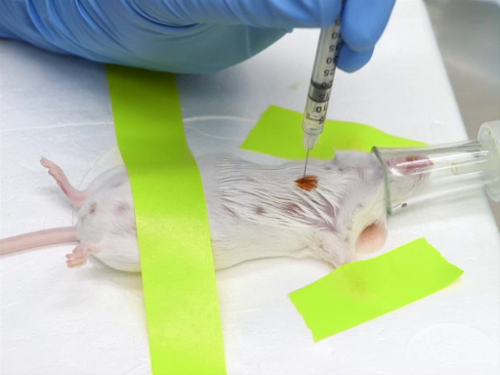
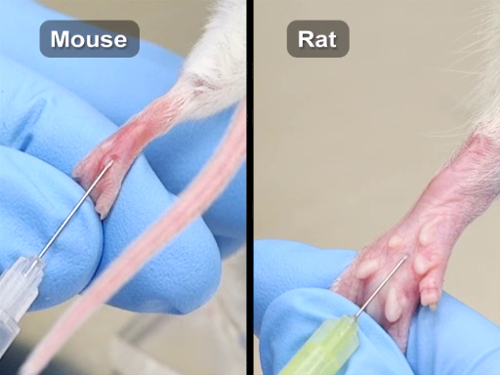
Compound Administration IV
Overview
Source: Kay Stewart, RVT, RLATG, CMAR; Valerie A. Schroeder, RVT, RLATG. University of Notre Dame, IN
There are many commonly used routes for compound administration in laboratory mice and rats. Protocols may, however, require the use of the less commonly used routes: intracardiac, footpad, and retro-orbital injections. Specialized training is essential for these procedures to be performed successfully. Justification for these routes may need to be provided to gain Institutional Animal Care and Use Committee (IACUC) approval.
Procedure
1. Intracardiac injection
- Landmarks and positioning: Position the mouse or rat either in right lateral recumbency (with the left side facing upward) or in dorsal recumbency, and identify the landmarks.
- Position the heart approximately level with the point of the elbow and just to the left of the sternum.
- Insert the needle between the ribs at the point of the elbow.
- In an animal in dorsal recumbency, insert the needle into the chest parallel to the table.
- In an anim
Application and Summary
The administration of compounds into animals can have a significant effect on both the wellbeing of the animal and the outcome of the experimental data and scientific value. The proper method of delivery is essential to the success of the experiment. Many factors must be considered to determine the best route, including the scientific aim of the study, the pH of the substance, the required dosage volume, the viscosity of the substance, and the wellbeing of the animals. Technical expertise is also a requirement for all in
References
- Morton, D.A., Jennings, M., Buckwell, A., Ewbank, R., Godfrey, C., Holgate, B., Inglis, I., James, R., Page, C., Sharman, I., Verschoyle, R., Westall, L., and Wilson, A.B. 2001. Refining procedures for the administration of substances Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Members of the Joint Working Group on Refinement. Laboratory Animals. 35. 1-41
- Prendiville , T.W., Qing, M., Lin, Z., Zhou, P., He, A., and Pu, W.T. 2014. Ultrasound-guided Transthoracic Intramyocardial Injection in Mice. Journal of Visualized Experiments. 90 | e51566.
- Yardeni, T., Eckhaus, M., Morris, H.D., Huizing, M., and Hoogstraten-Miller, S. 2001. Retro-orbital injection in mice. Lab Animal. 40:5. 155-171.
- Steel, C., Stephens, A., Hahto, S., Singletary, S., Ciavarra, R. 2008. Comparison of the lateral tail vein and the retro-orbital sinus as routes of intravenous drug delivery in a transgenic mouse model. Lab Anim. 37. 26-31.
- Timm, K.I. 1989. Orbital venous anatomy of the Mongolian gerbil, with comparison to the mouse, hamster, and rat. Laboratory Animal Science. 39:3. 262-265.
- Kamala, T. 2007. Hock immunization: a humane alternative to mouse footpad injections. Journal of Immunological Methods. 328. 204-214.
Tags
Skip to...
Videos from this collection:

Now Playing
Compound Administration IV
Lab Animal Research
52.0K Views

Rodent Handling and Restraint Techniques
Lab Animal Research
175.4K Views

Basic Care Procedures
Lab Animal Research
28.1K Views

Fundamentals of Breeding and Weaning
Lab Animal Research
35.8K Views

Rodent Identification I
Lab Animal Research
55.0K Views

Rodent Identification II
Lab Animal Research
25.7K Views

Compound Administration I
Lab Animal Research
101.1K Views

Compound Administration II
Lab Animal Research
35.1K Views

Compound Administration III
Lab Animal Research
31.6K Views

Blood Withdrawal I
Lab Animal Research
172.4K Views

Blood Withdrawal II
Lab Animal Research
73.7K Views

Anesthesia Induction and Maintenance
Lab Animal Research
50.9K Views

Considerations for Rodent Surgery
Lab Animal Research
22.5K Views

Diagnostic Necropsy and Tissue Harvest
Lab Animal Research
58.3K Views

Sterile Tissue Harvest
Lab Animal Research
34.9K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved