Quenching and Boiling
Source: Alexander S Rattner, Sanjay Adhikari, and Mahdi Nabil; Department of Mechanical and Nuclear Engineering, The Pennsylvania State University, University Park, PA
Controlled heating followed by rapid cooling is an important element of many materials processing applications. This heat-treating procedure can increase material hardness, which is important for cutting tools or surfaces in high wear environments. The rapid cooling stage is called quenching, and is often performed by immersing materials in a fluid bath (often water or oil). Quenching heat transfer can occur due to forced convection - when the action of rapidly moving material through coolant drives the heat transfer process, and due to free convection - when the reduced density of hot fluid near the material surface causes buoyancy-driven circulation and heat transfer. At high material temperatures, the coolant can boil, leading to increased heat transfer effectiveness. However, when extremely hot materials are quenched, they can be blanketed in relatively low thermal conductivity coolant vapor, leading to poor heat transfer.
In this experiment, quenching heat transfer will be measured for a heated copper cylinder, which is representative of small heat-treated parts. The transient sample temperature profile will be measured during quenching and compared with theoretical results for free convection heat transfer. Boiling phenomena will also be investigated qualitatively.
NOTE: This experiment uses flame heating. Ensure that a fire extinguisher is on hand and that no flammable materials are near the experiment. Follow all standard precautions for fire safety.
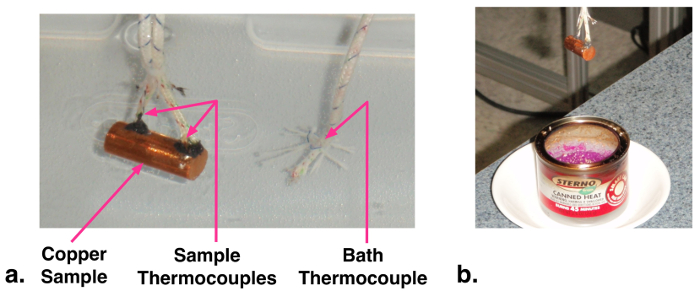
1. Fabrication of sample for quenching (see photograph, Fig. 1)
- Cut a small length (~24 mm) of 9.53 mm diameter copper rod. Drill two small holes (1.6 mm diameter) about halfway into the rod near the two ends. These holes will be the thermocouple wells. As the holes and thermocouples are relati
Photographs of boiling at different initial sample temperatures (Ts,0) are presented in Fig. 2. At Ts,0 = 150°C vapor bubbles form and stay attached to the sample. At Ts,0 = 175°C bubbles detach and float into the water. At 200°C, more bubbles are generated, and further increases are observed at higher temperatures. Boiling crisis type events (e.g., whole sample being surrounded by persistent vapor) are no
This experiment demonstrated the process of transient heat transfer during quenching. The temperature of a material sample was tracked as it was rapidly cooled in a water bath. The convection coefficients and temperature profiles over time were compared with theoretical values for free convection cooling. Boiling phenomena were also discussed and observed for high initial sample temperatures. Information from such experiments and demonstrated modeling approaches can be applied to understand and design heat transfer proce
Skip to...
Videos from this collection:

Now Playing
Quenching and Boiling
Mechanical Engineering
7.7K Views

Buoyancy and Drag on Immersed Bodies
Mechanical Engineering
29.6K Views

Stability of Floating Vessels
Mechanical Engineering
22.0K Views

Propulsion and Thrust
Mechanical Engineering
20.7K Views

Piping Networks and Pressure Losses
Mechanical Engineering
57.5K Views

Hydraulic Jumps
Mechanical Engineering
40.7K Views

Heat Exchanger Analysis
Mechanical Engineering
27.9K Views

Introduction to Refrigeration
Mechanical Engineering
24.5K Views

Hot Wire Anemometry
Mechanical Engineering
15.5K Views

Measuring Turbulent Flows
Mechanical Engineering
13.4K Views

Visualization of Flow Past a Bluff Body
Mechanical Engineering
11.1K Views

Jet Impinging on an Inclined Plate
Mechanical Engineering
10.7K Views

Conservation of Energy Approach to System Analysis
Mechanical Engineering
7.2K Views

Mass Conservation and Flow Rate Measurements
Mechanical Engineering
22.5K Views

Determination of Impingement Forces on a Flat Plate with the Control Volume Method
Mechanical Engineering
25.9K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved
 = (Ts,j+1-Ts,j)/(tj+1-tj) (values will be negative). Here, tj is the time of each logged reading. It may be helpful to smooth these cooling rate curves by performing a running average with a sample window of 2-3 readings.
= (Ts,j+1-Ts,j)/(tj+1-tj) (values will be negative). Here, tj is the time of each logged reading. It may be helpful to smooth these cooling rate curves by performing a running average with a sample window of 2-3 readings.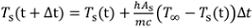 (3)
(3)