Flow Cytometry and Fluorescence-Activated Cell Sorting (FACS): Isolation of Splenic B Lymphocytes
Source: Perchet Thibaut1,2,3, Meunier Sylvain1,2,3, Sophie Novault4, Rachel Golub1,2,3
1 Unit for Lymphopoiesis, Department of Immunology, Pasteur Institute, Paris, France
2 INSERM U1223, Paris, France
3 Université Paris Diderot, Sorbonne Paris Cité, Cellule Pasteur, Paris, France
4 Flow Cytometry Platfrom, Cytometry and Biomarkers UtechS, Center for Translational Science, Pasteur Institute, Paris, France
The overall function of the immune system is to defend the body against infectious organisms and other invaders. White blood cells, or leukocytes, are the key players of the immune system. Upon infection, they are activated and initiate an immune response. Leukocytes can be divided into various sub-populations (e.g., myeloid cells, lymphocytes, dendritic cells) based on different parameters that can be biological, physical, and/or functional (e.g., size, granularity, and secretion). One way to characterize leukocytes is through their surface proteins, which are mainly receptors. Each leukocyte population expresses a specific combination of receptors (e.g., cytotoxic, activating, migration receptors) that can define subsets among populations. As the immune system encompasses a wide range of cell populations, it is essential to characterize them to decipher their participation in the immune response.
Flow cytometry (FC or FCM) is a widely used method for analyzing the expression of cell surface and intracellular molecules, characterizing and defining different cell types in a heterogenous cell mixture. Flow cytometers are composed of three main sub-systems: fluidics, optics, and electronics. The fluidics system transports the cells in a stream such that they pass in front of a laser one by one. The optics system consists of light sources (lasers) to illuminate the particles, optical filters to direct the resulting light, and fluorescent signals to appropriate detectors. Finally, the electronics system converts the detected light signals into electronic signals that can be processed by the computer. As an individual cell passes in front of the laser beam, it scatters light. A detector in front of the beam measures forward scatter (FS) and several detectors to the side measure side scatter (SC). The FS correlates with cell size and SC is proportional to the granularity of the cells. In this manner, cell populations can often be distinguished based on differences in their size and granularity alone.
In addition to analyzing a cell's size, shape, and complexity, flow cytometry is widely used for detecting the expression of cell surface receptors (1). This is accomplished by using fluorochrome-labelled monoclonal antibodies which bind to known cell-specific receptors. Upon excitation, these bound fluorochromes emit a light of specific wavelength, called emission wavelength, which can be detected and scored. Fluorescence measurements provide quantitative and qualitative data about fluorochrome-labeled cell surface receptors. Hematologists were first to use FC for the therapeutic follow up of immune cell populations (2). Now, it is used for a wide range of applications such as immunophenotyping, cell viability, gene expression, cell counting, and GFP analysis.
FACS (Fluorescent Activated Cell Sorter) is a specialized type of flow cytometry, which sorts a population of cells into subpopulation using fluorescent labelling. Just like conventional flow cytometry, first FS, SC, and fluorescent data are collected. Then, the machine applies a charge (negative or positive) and an electrostatic deflection system (electromagnets) facilitates the collection of charged droplets containing cells into appropriate tubes.
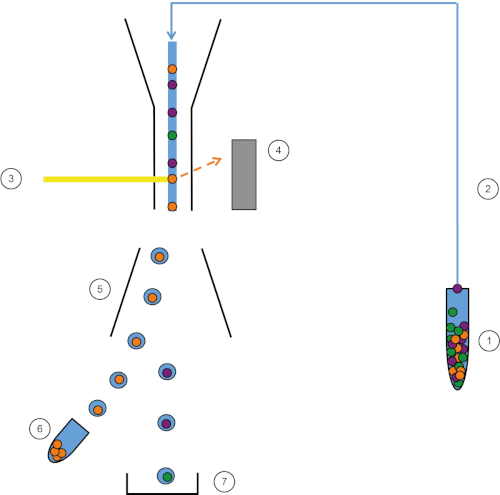
Figure 1: Schematic representation of FACS. Sample (1) is aspirated in the FACS (2) and passed in front of the laser (3). Cell fluorescence is sensed by fluorescence detectors (4). Finally, cells are incorporated in droplets and the cells of interest are deflected by deflection plates (5) and collected in a collection tube (6). The remaining cells go into the trash (7). Please click here to view a larger version of this figure.
The sorting aspect of the FACS presents many advantages. Many tests can help understand the role of specific cells in immune system, such as analyses of gene expression like RT-qPCR, cell cycle, or cytokine secretion. However, cells should be purified upstream to obtain clear and specific results. Here, FACS comes in useful and the desired cells can be sorted with great purity, yielding highly reliable and reproducible results. FACS can also be used to sort cells based on nuclear or other intracellular staining and according to the presence, absence, and density of surface receptors. FACS is now a standard technique for the purification of subpopulations of cells and has the ability sort up to four populations simultaneously.
This lab exercise demonstrates how to isolate splenic leukocytes and then how to specifically sort B lymphoid cells from the splenic leukocyte cell mixture using FACS.
1. Preparation
- Before beginning, put on laboratory gloves and the appropriate protective clothing.
- Sterilize all the dissection tools, first with a detergent and then with 70% ethanol and then dry thoroughly.
- Prepare 50mL of Hank's balanced salt solution (HBSS) containing 2% fetal calf serum (FCS).
2. Dissection
- Using a carbon dioxide delivery system, euthanize the mouse by hypoxia. Secure the euthanized m
In this protocol, we purified splenic B lymphocytes using FACS technology. We first isolated leukocytes from the spleen and stained them. Using a combination of B cell surface markers, we created a gating strategy to sort them (Figure 2, top panel). At the end of the experiment we verified if cells in the collection tube were B cells via a "purity test". We kept the same gating strategy and observed that more than 98% of the cells were indeed B cells (Figure 2, bottom panel). Thus
Flow cytometry is a first-hand technique to characterize and sort immune cell populations with a high degree of purity. It is primordial tool in research field as it allows enrichment of specific cell populations and to decipher the immune response to pathogens. With the increase in number of available fluorochromes and cytometers, the number of detectable parameters is highly increased. As a result, bioinformatic analysis of FACS data has begun to emerge and have opened new horizons to flow cytometry (3). Flow cytometry
- Lanier, L. L. Just the FACS. The Journal of Immunology, 193 (5), 2043-2044 (2014).
- Walker, J. M. Epiblast Stem Cells IN Series Editor.
- Tung, J. W., Heydari, K., Tirouvanziam, R., Sahaf, B., Parks. D. R., Herzenberg, L. A., and Herzenberg. L. A. Modern Flow Cytometry: A Practical Approach. Clinics in Laboratory Medicine. 27 (3), 453-468 (2007).
- Walker, J. M. Tumor Angiogenesis Assays IN Series Editor.
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved