The relative amount of a given solution component is known as its concentration. Often, though not always, a solution contains one component with a concentration that is significantly greater than that of all other components. This component is called the solvent and may be viewed as the medium in which the other components are dispersed or dissolved. Solutions in which water is the solvent are, of course, very common on our planet. A solution in which water is the solvent is called an aqueous solution.
A solute is a component of a solution that is typically present at a much lower concentration than the solvent. Solute concentrations are often described with qualitative terms such as dilute (of relatively low concentration) and concentrated (of relatively high concentration).
Concentrations may be quantitatively assessed using a wide variety of measurement units, each convenient for particular applications. Molarity (M) is a useful concentration unit for many applications in chemistry. Molarity is defined as the number of moles of solute in exactly 1 liter (1 L) of the solution and has the units of ‘mol/L’.
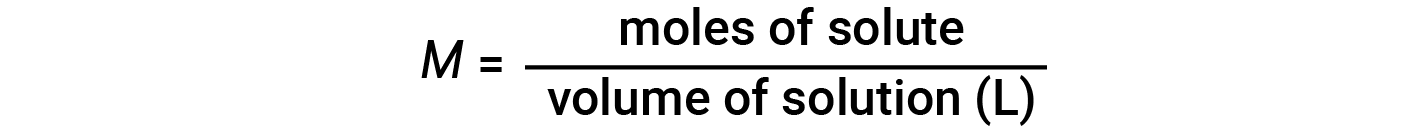
Note that in the molarity equation, the volume of solution, and not the volume of solvent, is used. This is because, depending on the nature of interactions between the solute and solvent, the solute can change the volume of the solution. Hence, in the molarity equation, we use the total solution volume (i.e., solvent volume + solute volume). Because solution volumes vary with temperature, molar concentrations will likewise vary. When expressed as molarity, the concentration of a solution with identical numbers of solute and solvent species will be different at different temperatures, due to the contraction/expansion of the solution.
Dilution of Solutions
Dilution is the process whereby a solution is made less concentrated (or more dilute) by the addition of solvent. For example, a glass of iced coffee becomes increasingly dilute, and less sweet, as the ice melts. In laboratories, solutions are often stored in their concentrated forms, called stock solutions. Solutions of lower concentrations are prepared from stock through dilution.
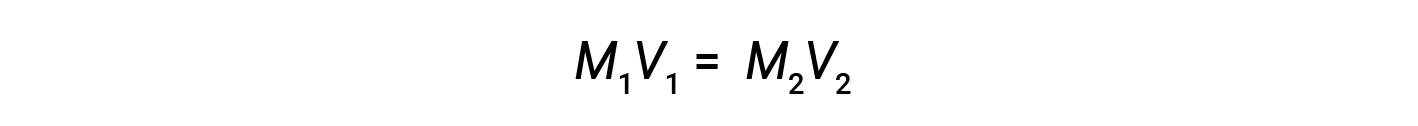
where M and V are concentration and volume, respectively, and the subscripts “1” and “2” refer to the solution before and after the dilution, respectively.
Now, since the product of molarity and volume equals moles, the number of moles before and after dilution stays the same.
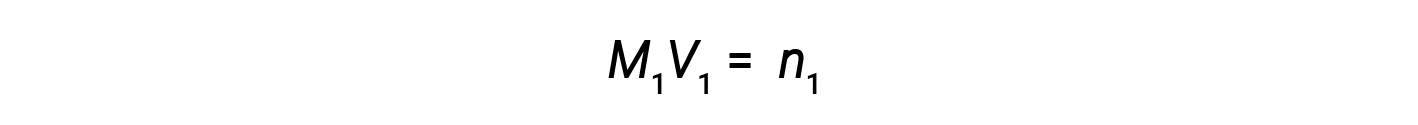
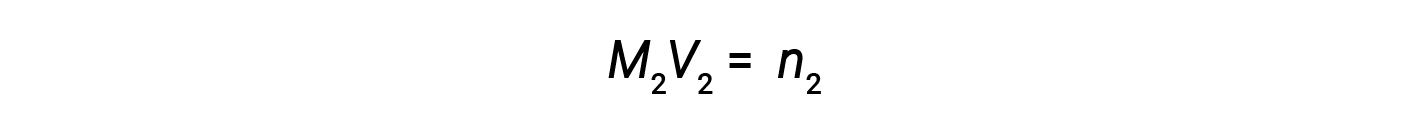
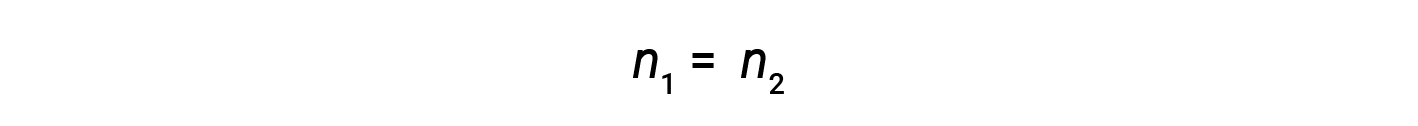
Thus, dilution does not change the amount of solute in the solution.
This text is adapted from OpenStax Chemistry 2e, Section 3.3: Molarity.
From Chapter 4:

Now Playing
4.5 : Solution Concentration and Dilution
Chemical Quantities and Aqueous Reactions
81.7K Views

4.1 : Reaction Stoichiometry
Chemical Quantities and Aqueous Reactions
58.9K Views

4.2 : Limiting Reactant
Chemical Quantities and Aqueous Reactions
52.5K Views

4.3 : Reaction Yield
Chemical Quantities and Aqueous Reactions
42.2K Views

4.4 : General Properties of Solutions
Chemical Quantities and Aqueous Reactions
27.8K Views

4.6 : Electrolyte and Nonelectrolyte Solutions
Chemical Quantities and Aqueous Reactions
59.6K Views

4.7 : Solubility of Ionic Compounds
Chemical Quantities and Aqueous Reactions
58.9K Views

4.8 : Chemical Reactions in Aqueous Solutions
Chemical Quantities and Aqueous Reactions
57.1K Views

4.9 : Precipitation Reactions
Chemical Quantities and Aqueous Reactions
47.9K Views

4.10 : Oxidation-Reduction Reactions
Chemical Quantities and Aqueous Reactions
60.5K Views

4.11 : Oxidation Numbers
Chemical Quantities and Aqueous Reactions
34.8K Views

4.12 : Acids, Bases and Neutralization Reactions
Chemical Quantities and Aqueous Reactions
52.4K Views

4.13 : Synthesis and Decomposition Reactions
Chemical Quantities and Aqueous Reactions
31.1K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved