The physical form of a substance changes on changing its temperature. For example, raising the temperature of a liquid causes the liquid to vaporize (convert into vapor). The process is called vaporization—a surface phenomenon. Vaporization occurs when the thermal motion of the molecules overcome the intermolecular forces, and the molecules (at the surface) escape into the gaseous state. When a liquid vaporizes in a closed container, gas molecules cannot escape. As these gas phase molecules move randomly about, they will occasionally collide with the surface of the condensed phase, and in some cases, these collisions will result in the molecules re-entering the condensed phase. The change from the gas phase to the liquid is called condensation.
Vaporization is an endothermic process. The cooling effect is evident after a swim or a shower. When the water on the skin evaporates, it removes heat from the skin and cools the skin. The energy change associated with the vaporization process is the enthalpy of vaporization, ΔHvap. For example, the vaporization of water at standard temperature is represented by:

The reverse of an endothermic process is exothermic. And so, the condensation of a gas releases heat:
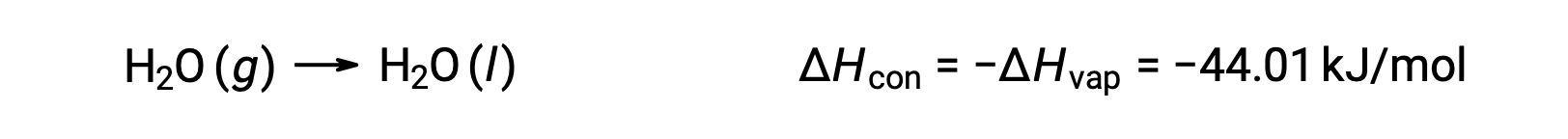
Vaporization and condensation are opposing processes; consequently, their enthalpy values are identical with opposite signs. While the enthalpy of vaporization is positive, the enthalpy of condensation is negative.
Different substances vaporize to different extents (depending on the strengths of their IMFs) and hence display different enthalpy of vaporization values. Relatively strong intermolecular attractive forces between molecules result in higher enthalpy of vaporization values. Weak intermolecular attractions present less of a barrier to vaporization, yielding relatively low values of enthalpies of vaporization.
This text is adapted from Openstax, Chemistry 2e, Section 10.3: Phase Transitions.
From Chapter 11:

Now Playing
11.7 : Phase Transitions: Vaporization and Condensation
Liquids, Solids, and Intermolecular Forces
16.7K Views

11.1 : Molecular Comparison of Gases, Liquids, and Solids
Liquids, Solids, and Intermolecular Forces
39.6K Views

11.2 : Intermolecular vs Intramolecular Forces
Liquids, Solids, and Intermolecular Forces
83.5K Views

11.3 : Intermolecular Forces
Liquids, Solids, and Intermolecular Forces
54.8K Views

11.4 : Comparing Intermolecular Forces: Melting Point, Boiling Point, and Miscibility
Liquids, Solids, and Intermolecular Forces
43.2K Views

11.5 : Surface Tension, Capillary Action, and Viscosity
Liquids, Solids, and Intermolecular Forces
27.1K Views

11.6 : Phase Transitions
Liquids, Solids, and Intermolecular Forces
18.4K Views

11.8 : Vapor Pressure
Liquids, Solids, and Intermolecular Forces
33.6K Views

11.9 : Clausius-Clapeyron Equation
Liquids, Solids, and Intermolecular Forces
54.5K Views

11.10 : Phase Transitions: Melting and Freezing
Liquids, Solids, and Intermolecular Forces
12.1K Views

11.11 : Phase Transitions: Sublimation and Deposition
Liquids, Solids, and Intermolecular Forces
16.4K Views

11.12 : Heating and Cooling Curves
Liquids, Solids, and Intermolecular Forces
21.7K Views

11.13 : Phase Diagrams
Liquids, Solids, and Intermolecular Forces
38.0K Views

11.14 : Structures of Solids
Liquids, Solids, and Intermolecular Forces
13.3K Views

11.15 : Molecular and Ionic Solids
Liquids, Solids, and Intermolecular Forces
16.3K Views
See More
Copyright © 2025 MyJoVE Corporation. All rights reserved