A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Preparation of Giant Vesicles Exhibiting Visible-light-induced Morphological Changes
Not Published
In This Article
Summary
The synthesis of ruthenium complex surfactants exhibiting photoisomerization in giant vesicles is described. The preparation and light irradiation of the giant vesicles are also described.
Abstract
We describe the preparation of giant vesicles that incorporate a photoresponsive ruthenium complex having two alkyl chains. The vesicles exhibited morphological changes when exposed to visible light. The ruthenium complex proximal-[Ru(L1)(L2)OH2](NO3)2, proximal-2 (L1 is 4'-decyloxy-2,2';6',2"-terpyridine, L2 is 2-(2'-(6'-decyloxy)-pyridyl)quinoline) was prepared by a thermal reaction of Ru(L1)Cl3 and L2, followed by removal of a chloride ligand. In an aqueous solution and vesicle dispersions, proximal-2 was reversibly photoisomerized to the distal isomer. Giant vesicles containing proximal-2 were prepared by hydration of phospholipid films containing proximal-2 in the dark at 80 °C. Giant vesicles were frequently found in the dispersions prepared from DOPC/proximal-2 rather than from DPPC/proximal-2 (DOPC is 1,2-dioleoyl-sn-glycero-3-phosphocholine, DPPC is 1,2-dipalmitoyl -sn-glycero-3-phosphocholine). The ratio of proximal-2 and DOPC in the vesicle preparation was varied from 5:100 to 20:100. The light-induced morphological changes were observed for proximal-2/DOPC in the presence of Na2SO4. However, they were highly suppressed in the presence of NaOH. Incubation of light-exposed vesicles at 45 °C in the dark induced reverse morphological changes. Morphological changes were observed under fluorescence microscopy using 635 nm (red) light. Rhodamine-DOPC [rhodamine-DOPC: 1,2-dioleoyl-sn-glycero-3-phos-phoethanolamine-N-(lissamine rhodamine B sulfonyl)] was used to fluorescently label the vesicles.
Introduction
Controlling the morphologies and shapes of macro- and meso-scale molecular assemblies by external stimuli has attracted considerable attention.1,2 In particular, the control of vesicle morphologies by remote stimuli such as light has potential applications for drug delivery.3 In this context, organic photochromic molecules with hydrophobic and hydrophilic moieties have been widely incorporated into liposomes and polymer vesicles.4,5,6,7,8 However, most of the assemblies require ultraviolet (UV) light to drive the morphological changes, and their applications are limited because UV light is strongly scattered in living tissues and induces DNA damage and cell death.
Alternatively, utilization of visible or near-infrared light in the phototherapeutic window (600-1000 nm) is more favorable because of abundant sunlight and its high transmission in tissues of living organisms. In this regard, ruthenium complexes with polypyridyl ligands are suitable visible-light-responsive surfactants. They exhibit a strong visible light absorption band (ε~104 M-1 cm-1) that induces ligand substitution9,10 and photoisomerization.11,12,13,14,15,16 Incorporation of the ruthenium complexes into vesicles will expand their applications because these complexes are also known as water oxidation catalysts17,18,19 and bioactive molecules.20,21 Recently, ruthenium complexes have been incorporated into vesicles.22,23,24 However, controlling morphologies of vesicles via visible-light absorption has remained challenging.
We have previously reported irreversible and reversible photoisomerization of mononuclear ruthenium aqua complexes having asymmetric bidentate ligands.25,26,27,28 Recently, we synthesized novel surfactants (proximal-2, see Figure 1) that exhibit visible-light photoisomerization equilibria with distal-2 by introducing an alkyl chain on each tridentate and bidentate ligand of the ruthenium aqua complex. Giant vesicles incorporating proximal-2 undergo morphological changes under the irradiation of visible light in the phototherapeutic window.29 Herein, we describe the detailed syntheses of ruthenium complexes and the preparation of giant vesicles. The protocols will enable researchers to prepare, characterize, and utilize light-responsive giant vesicles.
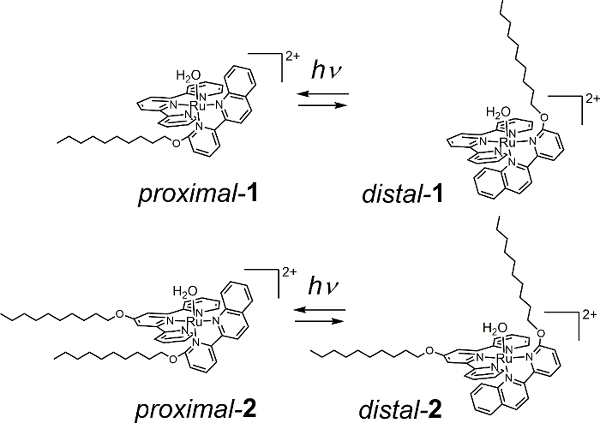
Figure 1: Ruthenium complex surfactants. Reversible photoisomerization equilibrium between proximal- and distal- type complex of 1 (top) and 2 (bottom). Please click here to view a larger version of this figure.
Protocol
NOTE: Ru(tpy)Cl330, L129, 2-(2'-(6'-chloro)-pyridyl)quinoline29, proximal- 129 were synthesized as previously described.
1. Synthesis of 2-(2'-(6'-decyloxy)-pyridyl)quinoline (L2)
- Add 2-(2'-(6'-chloro)-pyridyl)quinoline (16.3 mg, 63 µmol), 1-decanol (0.1 mL), dimethyl sulfoxide (1 mL), KOH (0.12 g) to a 50 mL round bottom flask equipped with a stir bar.
- Heat and stir the reaction mixture in an 80 °C oil bath for 4 h.
- Transfer the reaction mixture to a separating funnel, and add chloroform (ca. 20 mL) and water (ca. 20 mL). Shake the funnel for 2-3 minutes and wait several hours for complete separation into two layers. Collect the bottom organic layer, add anhydrous magnesium sulfate to absorb the water in the chloroform, filter with folded filtration paper, and remove the solvent in a rotary evaporator at 40 °C to obtain the oily crude product.
- Purify the product with silica gel chromatography (1.5 cm×20 cm) using a mixed solvent (AcOEt/hexane/CHCl3, 1:5:5, v/v/v) as an eluent.29 The product band emits blue light in the silica gel under UV light (254 nm).
- Collect the fractions of the blue band eluted from the column to the glass vials, and remove the solvent in a rotary evaporator at 40 °C. Check the product purity with 1H and 13C NMR in CDCl3 referenced with tetramethylsilane (TMS). The oily product contains a small amount of 1-decanol as impurity.29
2. Synthesis of proximal -2
- Synthesis of [Ru(L1)Cl 3 ]
- Add RuCl3·3H2O (60 mg, 0.23 mmol), L1 (80 mg, 0.21 mmol), and ethanol (EtOH, 40 mL) to a 50 mL round bottom flask equipped with a stir bar.
- Reflux and stir the reaction mixture in an oil bath for 4 h.
- Collect the yellow-brown precipitate with vacuum filtration, and wash with water (ca. 5 mL).
- Dry the product in vacuum for a yield of approximately 98 mg (80% yield).
- Synthesis of [Ru(L1)(L2)Cl]Cl
- Add [Ru(L1)Cl3] (47.2 mg, 0.078 mmol), triethylamine (0.2 mL), EtOH (12mL), water (4mL), LiCl (50 mg), and purified product of L2 synthesized from 0.10 mmol of 2-(2'-(6'-chloro)-pyridyl)quinoline to a 50 mL round bottom flask equipped with a stir bar.
- Reflux the reaction mixture in an oil bath for 4 h.
- Filter the purple solution with diatomite (ca. 2 g) on the filter paper in a glass funnel to remove unreacted [Ru(L1)Cl3].
- Reduce the solvent to ca. 3 mL in a rotary evaporator at 45 °C, collect the purple precipitate by vacuum filtration, and wash with diethyl ether.
- Purify the crude solid (44.2 mg) with size exclusion chromatography on a dextran gel, using methanol as eluent (column length: 20 cm).29 Collect fractions of the second purple band eluted from the column to glass vials. Spot the eluted fractions on the thin layer chromatography plate (ca. 1 µL for each spot). Check the purity using a mixed eluent (MeOH/saturated aqueous solution of NaCl, 30:1, v/v). Repeat the purification process two or three times.
- Remove the solvent on a rotary evaporator at 45 °C and dry in vacuum to obtain 30.1 mg of the product (39% yield). Check the purity with by 1H and 13C NMR in CDCl3 referenced with TMS.29
- Synthesis of proximal- [Ru(L1)(L2)OH 2 ](NO 3 ) 2 ( proximal- 2)
- Add proximal-[Ru(L1)(L2)Cl]Cl (16.3 mg, 0.017 mmol), water (3 mL), acetone (10 mL), and an aqueous solution of 0.1 M AgNO3 (0.60 mL, 0.060 mmol) to a 50 mL round bottom flask equipped with a stir bar. Cover the flask with aluminum foil
- Reflux the reaction mixture in an oil bath for 2 h in the dark.
- Filter the purple solution with diatomite (ca. 2 g) on the filter paper in a glass funnel.
- Reduce the solvent to ca. 3 mL in a rotary evaporator at 45 °C, collect the purple solid, and wash it with water.
- Dry in vacuum to obtain 12.6 mg of the product (75% yield). Check the purity with 1H and 13C NMR in the mix solvent of d-acetone and D2O (1:1, v/v) referenced with TMS.29
3. Standard conditions for preparation of vesicles
- To prepare 0.5 mM stock solution A, dissolve 4.0 mg of proximal- 2 in 8.0 mL of chloroform. Store the stock solution in the dark and refrigerate.
- To prepare 1.0 mM stock solution B, dissolve 15.7 mg of DOPC in 20.0 mL of chloroform.
- To prepare 0.1 mM stock solution C, dilute 1 mg/mL solution of rhodamine-DOPC with 6.6 mL of chloroform.
- Mix 40 µL of stock solution A and 100 µL of stock solution B in an amber glass vial. For the fluorescence microscopy experiments, add 100 µL of stock solution C.
- Seal the vial with a rubber septum equipped with a nitrogen inlet and outlet, and dry the solution under nitrogen flow overnight.
- Remove the septum and heat the vial in an 80 °C oven for 30 min.
- Add 0.1 mL of pure water to the vial. Seal and incubate the vial at 80 °C overnight. The lipid film is gradually hydrated to yield a reddish-purple vesicle dispersion that settles to the bottom of the vial.
- Store the vial in a refrigerator in the dark. The vesicle dispersion should be used within a week.
4. Preparation of Plates
- Rinse glass plates with ethanol and acetone, sonicate in ethanol for 5 min, and dry at 50 °C for 20-30 min.
- Cut a silicon film (thickness = 0.2 mm) into a 20 mm×20 mm square with a knife.
- Make a 5 mm hole with a hole punch at the center of the film, and remove plastic covers.
- Wet one side of the silicon film with diluted detergent (0.3 %) and then wipe it with cleaning tissue.
- Attach the edge of the film to a glass plate, and slowly lay the film in order to extrude the bubbles.
- Slowly shake the amber vial containing the vesicles, and with a micropipette transfer a small drop (diameter ~1 mm) to the center of the hole on the glass plate.
- Place a cover glass (18 mm × 18 mm) on the vesicle dispersion.
5. Morphological changes of giant vesicles under visible light irradiation
- Perform experiments in the dark at a constant temperature of 25 °C.
- Put the glass plate with sample droplets under a digital microscope (700X), and acquire images.
- Expose the sample plate with emission from a halogen lamp (distance from the plate: 2.5 cm) at a constant intensity of 120 mWcm-2.
6. Morphological changes of giant vesicles under red light irradiation
- Perform experiments in the dark at a constant temperature of 25 °C.
- Put the glass plate with sample droplets containing DOPC, proximal-2, and rhodamine-DOPC under a confocal microscope.
- Acquire images with a confocal microscope. Transmit excitation light (559 nm) and emission light (575 nm) through the same objective.
- Turn on the LED laser (635 nm), and adjust its intensity to 20 mW.
Results
We obtained high-purity proximal-2 to form spherical and giant multilamellar vesicles (proximal-2/DOPC, proximal-2: DOPC=20:100) 15-µm average diameters (see Table 1).29 Several layers were found inside the vesicles (Figures 2A and 2C). The inner spheres of the vesicles in Figures 2B, and 2D were darker than the outer spheres because of the concentric lipid layers. T...
Discussion
The ruthenium chloro complex proximal-[Ru(L1)(L2)Cl]+ was prepared by thermal synthesis of Ru(L1)Cl3 and a bidentate ligand L2 in the presence of triethylamine. The proximal isomer was the major product and a distal isomer and Ru(L1)22+ was a minor impurity. The crude product was purified with size-exclusion chromatography using methanol as an eluent. Coordinating solvents, such as wat...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements.
Materials
| Name | Company | Catalog Number | Comments |
| Triethylamine | Wako Pure Chemical Industries, Ltd. | 202-02646 | |
| Lithium Chloride | Wako Pure Chemical Industries, Ltd. | 125-01161 | |
| Chloroform | Kanto Chemical Co. Ltd. | 07278-03 | Used for vesicle preparation |
| Chloroform | Junsei Chemical Co. Ltd. | 28560-0382 | Used for ligand synthesis |
| Acetone | Junsei Chemical Co. Ltd. | 11265-0382 | |
| Ethanol | Junsei Chemical Co. Ltd. | 17065-0382 | |
| Ethyl Acetate | Junsei Chemical Co. Ltd. | 67150-0382 | |
| Hexane | Junsei Chemical Co. Ltd. | 31055-0382 | |
| Silica gel | Kanto Chemical Co. Ltd. | 37558-79 | 100-210 μm |
| 1-decanol | Tokyo Chemical Industry Co., Ltd. | D0031 | 25 mL |
| Potassium hydroxide | Kanto Chemical Co. Ltd. | 32344-00 | |
| Sodium hydrixude | Wako Pure Chemical Industries, Ltd. | 197-02125 | |
| Dimethyl sulfoxide (DMSO) | Kanto Chemical Co. Ltd. | 10378-00 | |
| d-DMSO | Sigma-Aldrich | 166290100 | |
| CD3OD | Kanto Chemical Co. Ltd. | 25221-43 | |
| d-Acetone | Kanto Chemical Co. Ltd. | 01054-43 | |
| D2O | Cambridge Isotope Laboratories, Inc. | DLM-4-100 | |
| Ruthenium chloride n-Hydrate | Wako Pure Chemical Industries, Ltd. | 183-00823 | |
| 2,2':6',2"-Terpyridine | Sigma-Aldrich | 234672-5G | |
| 0.1 mol/L Silver nitrate solution | Wako Pure Chemical Industries, Ltd. | 192-00855 | |
| Sodium sulfate | Kanto Chemical Co. Ltd. | 37280-00 | |
| 1,2 Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) | Wako Pure Chemical Industries, Ltd. | 160-12781 | 100 mg, stored at -20°C |
| 1,2 Dioleoyl-sn-glycero-3-phosphocholine (DOPC) | Sigma-Aldrich | P6354-100mg | 100 mg, stored at -20°C |
| 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(carboxyfluorescein) (ammonium salt) | Avanti Polar Lipids, Inc. | Avanti 810332p | 5 mg, stored at -20°C |
| 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (ammonium salt) | Avanti Polar Lipids, Inc. | Avanti 810150c | 1 mg, stored at -20°C |
| Dextran gel | GE healthcare Japan | 17009010 | Sephadex LH-20 |
| Amber glass vial | Maruemu | 0407-06 | |
| Septum | Sigma-Aldrich | Z564648-100EA | |
| Heater | Advantech | DRM 320 DB | |
| Silicon film | AS ONE | 6-9085-03 | Thickness: 0.2 mm |
| Slide glass | Matsunami | S003130 | 76×26 mm, thickness: 0.8-1.0 mm |
| Cover glass | Matsunami | C218181 | 18×18 mm, thickness: 0.12-0.17 mm |
| Transfer pipette | Brand GMBH | 704774 | |
| Round-bottom flask | Vidtech | 1500-05 | |
| Sonicator | AS ONE | 1-4591-32 | |
| Optical power meter | OPHIR | ORION/PD P/N 1Z01803 | |
| Oil bath | Riko | MH-3D | |
| Magnetic stirrer | Riko | MSR-10 | |
| Diatomite | Wako Pure Chemical Industries, Ltd. | 537-02305 | Celite 545 |
| Evaporator | Yamato | RE-52 | |
| Glass funnel | Kiriyama | SB-21 | 10 mL, 21 mmφ |
| Bell jar | Kiriyama | VKB-200 | |
| Filter paper | Kiriyama | No.4 | 21 mmφ |
| Optical microscope | KEYENCE | VHX-5000 | |
| Confocal fluorescence microscope | Olympus | FV-1000 |
References
- Natansohn, A., Rochon, P. Photoinduced Motions in Azo-Containing Polymers. Chem. Rev. 102 (11), 4139-4176 (2002).
- Ichimura, K., Oh, S. -. K., Nakagawa, M. Light-Driven Motion of Liquids on a Photoresponsive Surface. Science. 288 (5471), 1624-1626 (2000).
- Shum, P., Kim, J. -. M., Thompson, D. H. Phototriggering of liposomal drug delivery systems. Adv. Drug Deliv. Rev. 53 (3), 273-284 (2001).
- Mabrouk, E., Cuvelier, D., Brochard-Wyart, F., Nassoy, P., Li, M. -. H. Bursting of sensitive polymersomes induced by curling. Proc. Natl. Acad. Sci. U. S. A. 106 (18), 7294-7298 (2009).
- Hamada, T., Sato, Y. T., Yoshikawa, K., Nagasaki, T. Reversible Photoswitching in a Cell-Sized Vesicle. Langmuir. 21 (17), 7626-7628 (2005).
- Diguet, A., et al. UV-Induced Bursting of Cell-Sized Multicomponent Lipid Vesicles in a Photosensitive Surfactant Solution. J. Am. Chem. Soc. 134 (10), 4898-4904 (2012).
- Hamada, T., Sugimoto, R., Vestergaard, M. d. C., Nagasaki, T., Takagi, M. Membrane Disk and Sphere: Controllable Mesoscopic Structures for the Capture and Release of a Targeted Object. J. Am. Chem. Soc. 132 (30), 10528-10532 (2010).
- Li, L., et al. Light-Switchable Vesicles from Liquid-Crystalline Homopolymer-Surfactant Complexes. Angew. Chem. Int. Ed. 51 (46), 11616-11619 (2012).
- Pinnick, D. V., Durham, B. Photosubstitution reactions of Ru(bpy)2XYn+ complexes. Inorg. Chem. 23 (10), 1440-1445 (1984).
- Rack, J. J., Winkler, J. R., Gray, H. B. Phototriggered Ru(II)−Dimethylsulfoxide Linkage Isomerization in Crystals and Films. J. Am. Chem. Soc. 123 (10), 2432-2433 (2001).
- Durham, B., Walsh, J. L., Carter, C. L., Meyer, T. J. Synthetic Applications of Photosubstitution Reactions of Poly(Pyridyl) Complexes of Ruthenium(II). Inorg. Chem. 19 (4), 860-865 (1980).
- Bonnet, S., Collin, J. -. P., Sauvage, J. -. P. Light-Induced Geometrical Changes in Acyclic Ruthenium(II) Complexes and Their Ruthena−Macrocyclic Analogues. Inorg. Chem. 46 (25), 10520-10533 (2007).
- Bonnet, S., Collin, J. -. P., Sauvage, J. -. P. Synthesis and Photochemistry of a Two-Position Ru(terpy)(phen)(L)2+ Scorpionate Complex. Inorg. Chem. 45 (10), 4024-4034 (2006).
- Miyazaki, S., Kojima, T., Fukuzumi, S. Photochemical and Thermal Isomerization of a Ruthenium(II)−Alloxazine Complex Involving an Unusual Coordination Mode. J. Am. Chem. Soc. 130 (5), 1556-1557 (2008).
- Padhi, S. K., Fukuda, R., Ehara, M., Tanaka, K. Photoisomerization and Proton-Coupled Electron Transfer (PCET) Promoted Water Oxidation by Mononuclear Cyclometalated Ruthenium Catalysts. Inorg. Chem. 51 (9), 5386-5392 (2012).
- King, A. W., Wang, L., Rack, J. J. Excited State Dynamics and Isomerization in Ruthenium Sulfoxide Complexes. Acc. Chem. Res. 48 (4), 1115-1122 (2015).
- Concepcion, J. J., Jurss, J. W., Templeton, J. L., Meyer, T. J. One Site is Enough. Catalytic Water Oxidation by [Ru(tpy)(bpm)(OH2)]2+ and [Ru(tpy)(bpz)(OH2)]2+. J. Am. Chem. Soc. 130 (49), 16462-16463 (2008).
- Duan, L., et al. A molecular ruthenium catalyst with water-oxidation activity comparable to that of photosystem II. Nat. Chem. 4 (5), 418-423 (2012).
- Boyer, J. L., et al. Effects of a Proximal Base on Water Oxidation and Proton Reduction Catalyzed by Geometric Isomers of [Ru(tpy)(pynap)(OH2)]2+. Angew. Chem. Int. Ed. 50 (52), 12600-12604 (2011).
- Howerton, B. S., Heidary, D. K., Glazer, E. C. Strained Ruthenium Complexes Are Potent Light-Activated Anticancer Agents. J. Am. Chem. Soc. 134 (20), 8324-8327 (2012).
- Albani, B. A., et al. Marked Improvement in Photoinduced Cell Death by a New Tris-heteroleptic Complex with Dual Action: Singlet Oxygen Sensitization and Ligand Dissociation. J. Am. Chem. Soc. 136 (49), 17095-17101 (2014).
- Bonnet, S., Limburg, B., Meeldijk, J. D., Gebbink, R. J. M. K., Killian, J. A. Ruthenium-Decorated Lipid Vesicles: Light-Induced Release of [Ru(terpy)(bpy)(OH2)]2+ and Thermal Back Coordination. J. Am. Chem. Soc. 133 (2), 252-261 (2011).
- Askes, S. H. C., Bahreman, A., Bonnet, S. Activation of a Photodissociative Ruthenium Complex by Triplet-Triplet Annihilation Upconversion in Liposomes. Angew. Chem. Int. Ed. 53 (4), 1029-1033 (2014).
- Koshiyama, T., et al. Regulation of a cerium(iv)-driven O2 evolution reaction using composites of liposome and lipophilic ruthenium complexes. Dalton Trans. 44 (34), 15126-15129 (2015).
- Yamazaki, H., Hakamata, T., Komi, M., Yagi, M. Stoichiometric Photoisomerization of Mononuclear Ruthenium(II) Monoaquo Complexes Controlling Redox Properties and Water Oxidation Catalysis. J. Am. Chem. Soc. 133 (23), 8846-8849 (2011).
- Hirahara, M., et al. Mechanisms of Photoisomerization and Water-Oxidation Catalysis of Mononuclear Ruthenium(II) Monoaquo Complexes. Inorg. Chem. 52 (11), 6354-6364 (2013).
- Hirahara, M., et al. New Series of Dinuclear Ruthenium(II) Complexes Synthesized Using Photoisomerization for Efficient Water Oxidation Catalysis. Inorg. Chem. 54 (15), 7627-7635 (2015).
- Hirahara, M., et al. Mechanisms and factors controlling photoisomerization equilibrium, ligand exchange and water oxidation catalysis of mononuclear ruthenium(II) complexes. Eur. J. Inorg. Chem. 2015, 3892-3903 (2015).
- Hirahara, M., et al. Visible-Light-Induced Morphological Changes of Giant Vesicles by Photoisomerization of a Ruthenium Aqua Complex. Chem. Eur. J. 22 (8), 2590-2594 (2016).
- Sullivan, B. P., Calvert, J. M., Meyer, T. J. Cis-trans isomerism in (trpy)(PPh3)RuC12. Comparisons between the chemical and physical properties of a cis-trans isomeric pair. Inorg. Chem. 19 (5), 1404-1407 (1980).
- Walde, P., Cosentino, K., Engel, H., Stano, P. Giant Vesicles: Preparations and Applications. ChemBioChem. 11 (7), 848-865 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved