Cyclic Voltammetry (CV)
Source: Laboratory of Dr. Kayla Green — Texas Christian University
A Cyclic Voltammetry (CV) experiment involves the scan of a range of potential voltages while measuring current. In the CV experiment, the potential of an immersed, stationary electrode is scanned from a predetermined starting potential to a final value (called the switching potential) and then the reverse scan is obtained. This gives a 'cyclic' sweep of potentials and the current vs. potential curve derived from the data is called a cyclic voltammogram. The first sweep is called the 'forward scan' and the return wave is called the 'reverse scan'. The potential extremes are termed the 'scan window'. The magnitude of reduction and oxidation currents and the shape of the voltammograms are highly dependent on analyte concentration, scan rates, and experimental conditions. By varying these factors, cyclic voltammetry can yield information regarding the stability of transition metal oxidation state in the complexed form, reversibility of electron transfer reactions, and information regarding reactivity. This video will explain the basic setup for a cyclic voltammetry experiment including analyte preparation and setting up the electrochemical cell. A simple cyclic voltammetry experiment will be presented.
1. Preparation of Electrolyte Solution
- Prepare an electrolyte stock solution (10 mL) composed of 0.1 M [Bu4N][BF4] in CH3CN.
- Place the electrolyte solution in the electrochemical vial, add a small stir bar, and place the cap onto the vial as shown in Figure 1.
- Check to ensure that the nitrogen lead is in the electrolyte solution. Stir and degas the electrolyte solution with a gentle stream of dry N2 gas (~10 min) to remove redox
A CV scan of ferrocene at 300 mV/s in acetonitrile was carried out and the corresponding voltammogram is shown in Figure 2.
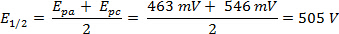
The ΔE can be derived from the data in Figure 2 based on the difference between Epa and Epc.
Log in or to access full content. Learn more about your institution’s access to JoVE content here
- Bard, A. J., Faulkner, L. A. Electrochemical methods: Fundamentals and Applications. 2nd ed. New York: Wiley; 833 p. (2001).
- Geiger, W. E., Connelly, N. G. Chemical Redox Agents for Organometallic Chemistry. Chem Rev. 96 (2), 877-910, (1996).
Skip to...
Videos from this collection:

Now Playing
Cyclic Voltammetry (CV)
Analytical Chemistry
122.3K Views

Sample Preparation for Analytical Characterization
Analytical Chemistry
82.5K Views

Internal Standards
Analytical Chemistry
201.9K Views

Method of Standard Addition
Analytical Chemistry
316.8K Views

Calibration Curves
Analytical Chemistry
781.2K Views

Ultraviolet-Visible (UV-Vis) Spectroscopy
Analytical Chemistry
610.5K Views

Raman Spectroscopy for Chemical Analysis
Analytical Chemistry
50.1K Views

X-ray Fluorescence (XRF)
Analytical Chemistry
25.1K Views

Gas Chromatography (GC) with Flame-Ionization Detection
Analytical Chemistry
277.6K Views

High-Performance Liquid Chromatography (HPLC)
Analytical Chemistry
377.6K Views

Ion-Exchange Chromatography
Analytical Chemistry
258.2K Views

Capillary Electrophoresis (CE)
Analytical Chemistry
91.9K Views

Introduction to Mass Spectrometry
Analytical Chemistry
110.1K Views

Scanning Electron Microscopy (SEM)
Analytical Chemistry
85.9K Views

Electrochemical Measurements of Supported Catalysts Using a Potentiostat/Galvanostat
Analytical Chemistry
51.0K Views
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved