COVID-19 / Coronavirus Outbreak: Performing a Nasal Swab Test on Patients inside a Rapidly Deployable Facility Optimized for Epidemics
While different types of diagnostic tests may be used to detect coronavirus disease, a nasopharyngeal swab test is the most common. The test must be performed by a trained healthcare provider. If cell culture is collected in a wrong manner, the diagnosis obtained may be incorrect.
The World Health Organization (WHO) recommends that providers use a sterile dacron or rayon swab with a flexible, plastic shaft for deep nasal sampling. Cotton, calcium alginate swabs, or swabs with wooden sticks may contain such compounds that can inactivate some viruses and inhibit the diagnostic polymerase chain reaction (PCR). The following protocol describes the proper procedure for collecting a nasopharyngeal swab sample.
- Escort the patient into the examination room area.
- Explain the process to the patient and obtain their consent to proceed.
- Open the swab package of a sterile swab and take out the swab.
- Tilt the patient’s head back slightly (about 70o) to straighten the passage from the front of the nose to the nasopharynx. Instruct the patient to close his or her eyes, then gently insert the swab along the nasal septum until resistance is felt (see Figure 1).
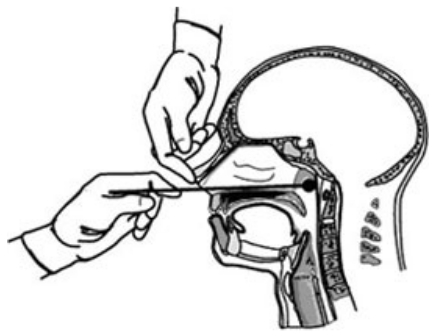
Figure 1. Image for the Surveillance of Vacine-Preventable Disease, 2015 (https://www.cdc.gov/pertussis/clinical/diagnostic-testing/specimen-collection.html#swab-testing)
- Rotate the swab several times for 10-15 sec to collect the sample material.
- Remove swab and insert the sample into a collection vial containing 1 – 3 mL of viral transport media.
- Break the swab handle at the marked breakpoint and close the vial.
- Label the vial with the following information:
- Collection date
- Onset date
- Patient age and sex
- Specimen type (e.g., nasal swab)
- Unique identifiers
- Other pertinent information
- Repeat the same procedure with the other nostril.
- Follow the manufacturer’s instructions to store the sample and transport it to an FDA approved diagnostic laboratory.
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved