A New Hybrid Quantitative Evaluation Model for Axillary Junctional Hemorrhage in Swine
* These authors contributed equally
In This Article
Summary
This study presents a hybrid quantitative model for axillary junctional hemorrhage in swine, enhancing pre-hospital hemostatic intervention evaluation.
Abstract
In this study, we developed and validated a hybrid quantitative model for simulating upper extremity junctional hemorrhage in swine, aiming to advance the development of pre-hospital hemostatic products. Utilizing 12 healthy 8-month-old male Yorkshire swine, we demonstrated the feasibility of a swine axillary artery injury model for evaluating hemostatic efficacy. Animals were divided into three groups to undergo volume-controlled hemorrhage (VCH), mimicking Class I-III hemorrhagic shock by withdrawing blood at different rates. Subsequent external compression was applied using a novel device consisting of a mechanical arm and an inflatable hemostatic balloon, achieving controlled pressure to enhance clot formation. Hemodynamic parameters, including heart rate and blood pressure, were continuously monitored, highlighting the impact of controlled hemorrhage and external compression on physiological responses. The findings suggest that the combination of VCH with targeted external compression effectively simulates clinical scenarios of axillary artery injury, providing a valuable model for testing hemostatic interventions in a controlled, standardized manner. This study underscores the potential of the model in facilitating the development and evaluation of new hemostatic agents and devices for managing junctional hemorrhages.
Introduction
The management of traumatic hemorrhage, especially in junctional regions where traditional tourniquets cannot be applied, presents a significant challenge in both military and civilian trauma care. Effective control of bleeding in areas such as the axillary and groin regions, characterized by complex vascular anatomy, is crucial for survival. Axillary artery injury is the main cause of massive hemorrhage at the upper extremity junctional site1. Dressings' packing and external compressing are the key steps in pre-hospital emergency hemorrhage control. The progress of new hemostatic dressings has greatly improved the hemostatic efficiency. However, external compression is essential for effective hemostasis in the early stage2,3. This study introduces a novel hybrid quantitative evaluation model using a swine model to simulate upper extremity junctional hemorrhage, aiming to advance pre-hospital hemostatic interventions.
Junctional injuries often require constant, direct manual pressure, which is challenging to achieve in prehospital settings due to limited personnel and unpredictable circumstances4. The rationale behind developing this technique lies in the limitations of existing methods for controlling junctional hemorrhage. The proposed hybrid quantitative evaluation model has a distinct advantage over alternative techniques. By using a swine model, which closely mimics human physiology, the study can provide more reliable and translatable results compared to a perfused cadaver model5.
Swine models are extensively utilized in vascular trauma research due to their physiological and anatomical similarities to humans, making them ideal for evaluating hemostatic agents and techniques6,7. The study's protocol meticulously combines volume-controlled hemorrhage (VCH) with external mechanical compression to accurately mimic the clinical scenario of axillary artery injury, thus addressing the urgent need for reliable hemostatic methods in junctional hemorrhage scenarios.
The integration of ultrasonography enhances the precision of hemorrhage control techniques and facilitates image interpretation, highlighting the interdisciplinary nature of modern trauma care. The development of a mechanical external compression device marks a significant advancement, offering a promising alternative to manual compression and traditional hemostatic methods. This research aims to determine the optimal pressure required for effective hemostasis in junctional areas, potentially filling a significant gap in trauma care literature.
Protocol
All experimental procedures received approval from the Laboratory Animal Welfare and Ethics Committee of the Army Medical University (AMUWEC20201513), adhering to the 3Rs ethical guideline, which advocates for the reduction, refinement, and replacement of animal use. A total of 12 healthy 8-month-old (49-56 kg) male Yorkshire swine were selected as the animal model and randomized into three groups (n=4/group). The weight and anatomical structure of Yorkshire swine are similar to those of humans, making them suitable for biomedical research.
1. Surgical preparation
NOTE: A typical experiment spans 120 min, starting with 30 min dedicated to surgical preparation. This is followed by a sequence of events at 10 min intervals, specifically defined as T0, T10, T20, T30, T40, T50, T60, T70, T80, and T90, ensuring meticulous timing throughout the process. After surgical setup, the protocol includes 20 min for (VCH) and 40 min for applying external compression. The experiment concludes with 30 min allocated for follow-up monitoring to assess the outcomes of the interventions (Figure 1).
- Animal preparation
- After arrival in the facility, house each swine in a separate room with a temperature of 24 °C and a humidity of 60%. Dry fast (abstaining from food but only on water) the swine for 12 h before the surgery to prevent nausea, vomiting, and aspiration.
- Preparation on the day of surgery
- Premedicate the swine by intramuscular injection of ketamine (20 mg/kg) and atropine (0.01 mg/kg) in the lateral region of the neck. Assess the level of anesthesia by jaw tone, and both pedal and palpebral reflex responses.
NOTE: Based on our experience, the anesthetic doses administered are sufficient to sedate the animal, and the use of atropine can prevent excessive salivation. However, if the time taken to transfer the animal from the housing to the operating room is too long, additional doses of ketamine (20 mg/kg) can be injected safely. Sedate the animal in accordance with the approved IACUC protocol. - Transfer the sedated animal to the operating room and secure its limb to an operating table in a supine position. Drape the operating table in a sterile fashion and equip it with a warming blanket.
- Puncture the ear vein with an intravenous indwelling needle. Attach a 60 inch extension line to the indwelling needle and connect the other end to a micro-pump. Induce general anesthesia and maintain by continuous infusion of fentanyl (4 µg/kg/h), midazolam (0.05 mg/kg/h), and propofol (10 mg/kg/h).
- Premedicate the swine by intramuscular injection of ketamine (20 mg/kg) and atropine (0.01 mg/kg) in the lateral region of the neck. Assess the level of anesthesia by jaw tone, and both pedal and palpebral reflex responses.
- Tracheostomy procedure
- Disinfect the neck area by scrubbing with povidone-iodine scrub and alcohol for at least 3 alternating rounds.
- Prior to making the incision, assess the depth of anesthesia through jaw tone and pedal reflex to ensure adequate anesthesia. Using a scalpel, make an 8 cm longitudinal skin incision from the thyroid cartilage to the manubrium.
- Dissect along the medial surfaces of the two sternohyoid muscles using blunt-tip surgical scissors, deepening the dissection and making a 1 cm incision at the trachea.
- Insert an appropriately sized tracheostomy tube (7mm-10mm) based on the size of the pig and connect the other side to the ventilator.
NOTE: In this model, the animals are ultimately humanely euthanized. The intubation procedure for pigs is relatively complex and time-consuming. To save surgical time and reduce the pain experienced by the animals, we opted for a rapid tracheostomy after anesthesia rather than intubation before anesthesia induction.
- Mechanical ventilation
- Set the ventilator to intermittent positive-pressure ventilation mode, with a tidal volume of 8 mL/kg, respiratory rate of 20 breaths per minute, and FiO2 of 30%. Adjust ventilator to maintain PCO2 between 35-45 mmHg. Adjust ventilator settings to maintain vital parameters, ensuring ETCO2 and SPO2 are within the appropriate range.
- Left carotid artery and jugular vein cannulation
- For the cannulation of the left carotid artery, employ the cut-down technique, necessitating a minimum of two operators for the procedure.
- Ensure the availability of crucial instruments for cannulation, including micro-scissors, sutures with needles, an 18G needle, a tube, a 5 French catheter sheath with an introducer, and a Seldinger guide wire.
- Using the incision made during tracheostomy, dissect the tissue lateral to the sternohyoid muscle to isolate the left carotid artery from the surrounding fascia.
- Use non-absorbable silk sutures to loop around the isolated segment of the left carotid artery before puncture.
- Utilize silk sutures to elevate the artery with one hand while puncturing it with the needle in the other. To verify the accuracy of the needle placement, administer 5 mL of 0.9% NaCl solution through the needle and observe for an arterial wall expansion. Following confirmation of the needle's correct positioning, advance the guide wire through the tube while concurrently retracting the needle.
- Extract the tube, then proceed to insert the sheath, utilizing the introducer guided over the wire. Following insertion, ensure the removal of both the introducer and the wire. Secure the sheath to the skin by suturing the flanges attached with 3-0 silk.
- Use the same technique to expose and cannulate the left jugular vein.
- Right carotid artery and jugular vein cannulation
- For the isolation of the right carotid artery and jugular vein, perform a lateral dissection adjacent to the sternohyoid muscle on the contralateral side.
- Place a 7.0 Fr central venous catheter with dual ports into the right jugular vein, and promptly link a transducer system for central venous pressure measurement.
NOTE: Catheter size may vary based on pig size. - Connect lactated Ringer's solution (7 mL/kg/h) to one port of the central line and administer a maintenance saline infusion (5 mL/kg/h) through the other port to mitigate the risk of catheter occlusion.
- Introduce a 4.0 Fr arterial catheter equipped with a thermistor into the right carotid artery, connecting it to the cardiac monitor while simultaneously connecting the monitor's venous measuring unit to the central venous transducer.
- Calibrate both the venous and arterial transducers to zero referencing the midaxillary line.
- Employ a thermal blanket to keep the animal's temperature within 37 °C -39 °C, with temperature monitoring facilitated by a thermistor at the arterial catheter's tip.
- Add mean arterial pressure to diastolic and systolic readings.
2. Volume-controlled hemorrhage
- Following the outlined protocol, T0 marks the start of the VCH, immediately after the initial 30 min surgical preparation period. Use baseline data collected at T0 for the initiation of hemorrhagic shock by withdrawing blood from the left jugular vein at specific average rates for each group: 0.33 mL/kg/min for Group I, 0.67 mL/kg/min for Group II, and 1 mL/kg/min for Group III, over a duration of 20 min.
- T20 signifies the conclusion of the VCH phase. At this juncture, ensure that groups I, II, and III attain hemorrhage levels of 10%, 20%, and 30%, respectively, relative to the total blood volume (TBV).
- Determine the TBV of the swine, expressed in mL, through manual calculation, employing an estimated blood volume parameter of 70 mL/kg1,2,3. It is imperative to maintain the specific average rate during the blood withdrawal process.
3. Axillary artery injury
- Disinfect the axilla by scrubbing with povidone-iodine scrub and alcohol at least 3 alternating rounds.
- Employ ultrasonography to map the surface projection of the axillary artery, utilizing Doppler sonography for the distinction of arterial, venous, and neural structures within the axillary fossa. Align the probe perpendicular to the dermal layer above the pectoralis muscle, which allows for the precise anatomical localization of the axillary artery.
- Mark a 10 cm arc-shaped incision as the surgical entry based on the anatomical landmarks established through ultrasonographic imaging (Figure 2A). The axillary artery lumen is anechoic, while the wall appears as a round, hyperechoic structure, and is surrounded by the hyperechoic brachial plexus.
- Apply a force of approximately 20 N with the probe and see the vein occlude upon compression with the ultrasound probe. The axillary sheath manifests as a hyperechoic, linear structure encasing the neurovascular bundle (Figure 2B).
- Incise the 10 cm arc-shaped marked incision on the skin with a scalpel. Continue to incise the subcutaneous tissue along the incision to expose the underlying structures. Partially remove the superficial and deep pectoralis muscles to expose the axillary sheath (Figure 2C).
NOTE: The axillary artery of the swine originates from the subclavian artery that is covered by the subclavian muscle. After reaching the upper edge of the deep pectoral muscle, it passes between the superficial and deep pectoral muscles and continues as the brachial artery. A deep axillary sheath surrounds the axillary artery, vein, and brachial plexus. - To expose the axillary artery, blunt dissect the axillary sheath with a micro-scissor and isolate a 6 cm segment of the artery from the surrounding vein and brachial plexus (Figure 2D).
- Upon isolation, loop two vascular blocking bands around the 6 cm segment to secure the vessel: place the red band at the proximal end and the green band at the distal end prior to the infliction of injury.
- Tie up the two vascular blocking bands to temporarily block the blood flow. If the vascular blocking bands are released, blood can be ejected from the vessel, simulating uncontrolled hemorrhage from axillary artery injury.
- Make a 2 mm transverse incision using a micro-scissor, which should be approximately 1/3 of the circumference of the vessel.
- Place 5 sheets of pre-weighted gauze (6 cm x 8 cm) over the incision on the axillary artery, with each sheet capable of absorbing approximately 30 mL of blood. Check the vascular blocking bands to ensure both are tied up and maintain tension to obstruct blood flow.
4. External compression
- Pre-set the external compression device
- Attach the mechanical arm to the side of the operating table. Connect the inflatable hemostatic balloon (Figure 3) to the end of the arm.
NOTE: The external compression device consists of three parts: a mechanical arm, an inflatable hemostatic balloon, and a pressure measurement system. - Immobilize the flexible film pressure sensor at the central point on the surface of the inflatable hemostatic balloon to measure the direct pressure. Connect the flexible film pressure sensor to an electrical wire, then link the wire to a data acquisition card.
- Attach the data acquisition card to a single-channel signal conditioning module and connect it to the data acquisition software.
NOTE: The pressure measurement system comprises a flexible film pressure sensor, an electrical wire, a data acquisition card, a single-channel signal conditioning conversion module (Figure 4), and data acquisition software. - After installation, open the software. Click on the Connected Device, select the Single-Channel Option, and choose 1000 Data Points Per Second from the sampling rate menu. For the input channel, select Band Display; for the output channel, select Output Display.
- Before recording, ensure the pressure measurement system is calibrated to zero. Click the Circular Red Button to start recording.
- Attach the mechanical arm to the side of the operating table. Connect the inflatable hemostatic balloon (Figure 3) to the end of the arm.
- To simulate external compression, target the mechanical arm on the axillary wound immediately after releasing the two vascular blocking bands. Manually inflate the hemostatic balloon to achieve local compression until the pressure reaches 210 mmHg. Record the pressure-time data and output in .CSV format in real time.
NOTE: Accurate pressure can be obtained through software processing after manually inflating the balloon. The pressure is measured in a range of 0-300 mmHg with a sampling rate of 1000 sampling points/second/channel. - Allow 40 min for external compression to enable the formation of local blood clots. Observe the device during compression to ensure that there is no displacement.
NOTE: In this experiment, effective compression was defined as achieving a blood loss under compression of less than 10% of the animal's body weight. - After the external compression, click the Red Square Button to stop data collection. Remove the device and weigh the gauze to calculate the blood loss under compression (Figure 5).
5. Follow-up and euthanasia
- Monitor the animal for 30 min after removal of the device.
- At the end of the experiment, increase the anesthetic infusion rates to a higher dose, such as fentanyl >10 µg/kg/h, propofol >20 mg/kg/h, and midazolam >0.1 mg/kg/h. Continue cardiac monitoring until the ECG shows no cardiac electrical activity and end-tidal CO2 tension reaches 0 mmHg.
- Confirm the absence of pulsatile flow on invasive arterial pressure monitoring and the absence of other vital signs.
NOTE: Follow the approved euthanasia procedure outlined in the IACUC protocol. A confirmatory method of euthanasia should also be performed, such as bilateral thoracotomy.
Representative Results
All the 12 animals survived till the end of the experiment. All the statistical analyses were processed by statistical analysis software. The hybrid quantitative model produces quantitative volume-controlled blood loss and consistent blood loss under compression. In Figure 6A, after VCH, the mean blood loss in groups I, II, and III was 354.2 mL, 714.4 mL, and 1064.0 mL respectively, accounting for 10%, 20%, and 30% of the TBV. These results demonstrate that this model can be used in various experimental setups, allowing for quantitative blood loss to be achieved through VCH. Figure 6B shows that there was no statistically significant difference in blood loss under compression among the three groups (p > 0.05). This consistent blood loss can be obtained by making an approximately 2 mm incision in the axillary artery using microscopic scissors, which is 1/3 of the circumference of the axillary artery. In Figure 6C, at the end of the experiment, the mean total blood loss for groups I, II, and III was 462.9 mL, 893.0 mL, and 1213.0 mL, respectively, achieving Class I, II, and III hemorrhagic shock, respectively.
The data depicted in Figure 7 elucidates the impact of controlled hemorrhage on hemodynamic parameters, indicating a marked increase in heart rate across all groups as a response to induced shock. The escalation in heart rate was most pronounced during the application of external compression and showed a decline upon its removal. The extent of heart rate elevation was directly proportional to the volume of blood withdrawn, with Group III exhibiting the most substantial increase, followed by Group II, and then Group I. This trend is quantitatively underscored by the maximal deviation in heart rate (ΔHR = HR_peak - HR_baseline), where Group III recorded a significantly higher change (Group I: 11.28 ± 1.2 bpm, Group II: 30.08 ± 2.7 bpm, Group III: 106.80 ± 7.2 bpm, p < 0.0001).
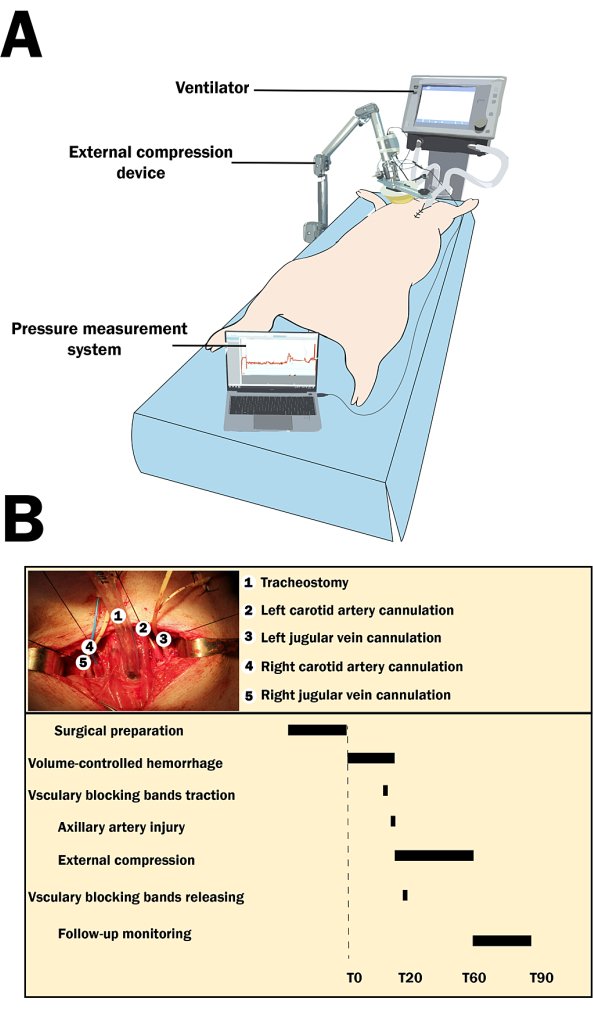
Figure 1: The experimental setup and the timeline of the experiment. (A) Experimental setting with the swine. (B) The surgical preparation steps, along with the timeline of the experiment. The timeline of the procedure extends over 120 min, beginning with a 30 min surgical preparation phase, followed by designated time points every 10 min (T0 to T90), where specific actions are taken, such as volume-controlled hemorrhage, vascular blocking bands traction and release, external compression, and follow-up monitoring. Please click here to view a larger version of this figure.
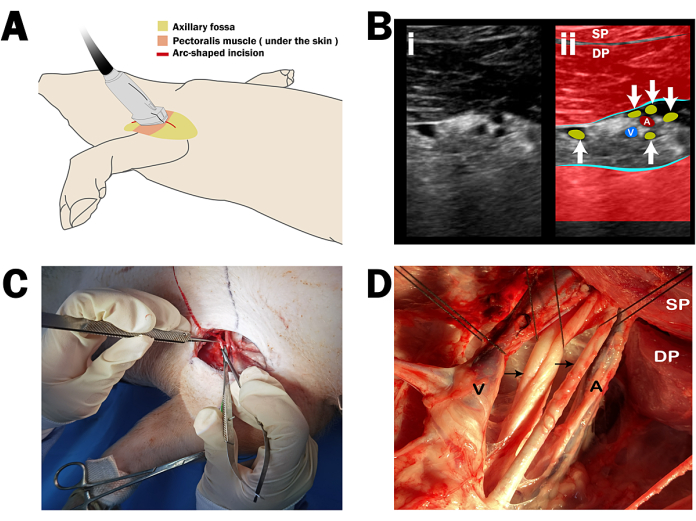
Figure 2: Ultrasound-guided dissection of axillary anatomy in swine. (A) Probe placement for the ultrasonographic identification of axillary structures, with a 10 cm arc-shaped incision marked for surgical entry. (B) Sonographic image detailing the axillary space at 6 cm depth; (i) Sonoanatomy and (ii) revised ultrasound anatomy. The linear transducer is oriented in a parasagittal plane. The axillary artery (A) and vein (V) are indicated alongside the brachial plexus (arrows), deep pectoral muscle (DP), and superficial pectoral muscle (SP). (C) Initial incision along the projected path of the axillary artery. (D) Exposure of the axillary artery and neurovascular bundle following the incision, with the superficial and deep pectoral muscles partially excised. Please click here to view a larger version of this figure.

Figure 3: Components of the external compression device. The inflatable hemostatic balloon. Please click here to view a larger version of this figure.
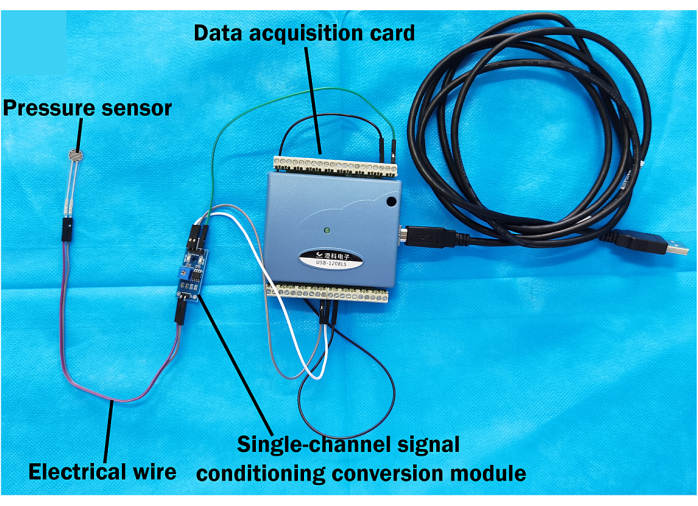
Figure 4: Pressure measurement system. Hardware components of the pressure measurement system, including the flexible film pressure sensor connected via electrical wire to a single-channel signal conditioning conversion module. The module is then connected to a data acquisition card, which interfaces with a computer. Please click here to view a larger version of this figure.
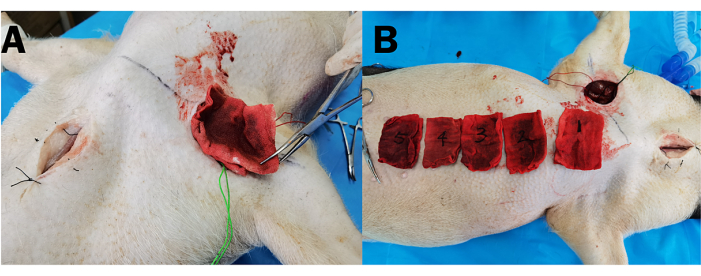
Figure 5: Assessment of blood loss under compression via gauze saturation. (A) Gauze extracted from the surgical wound after external compression. (B) The blood-soaked gauze aligned for pre-weighing to quantify blood loss under compression. Please click here to view a larger version of this figure.
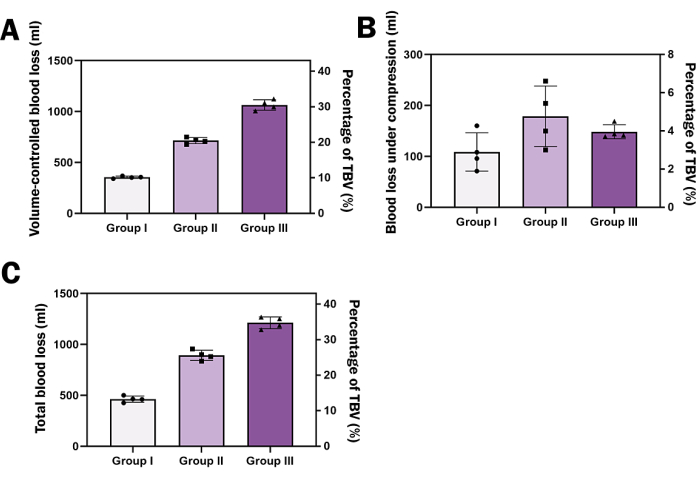
Figure 6: Assessment of blood loss and body weight. (A) Bar graph depicting volume-controlled blood loss as a percentage of total blood volume (TBV) for three different groups. (B) Bar graph showing blood loss under compression with no significant difference among the groups. (C) Bar graph representing total blood loss by the end of the experiment corresponding to three classes of hemorrhagic shock. The left Y-axis represents blood loss, while the right Y-axis represents the percentage of TBV. Each group has a sample size of N=6. All continuous data were expressed as mean ± standard deviation, and significance was set at 95% confidence intervals (C.I.s). Repeated Measures One-way analysis of variance (ANOVA) was employed to determine the effect of hemorrhage volume on hemodynamic parameters. Please click here to view a larger version of this figure.
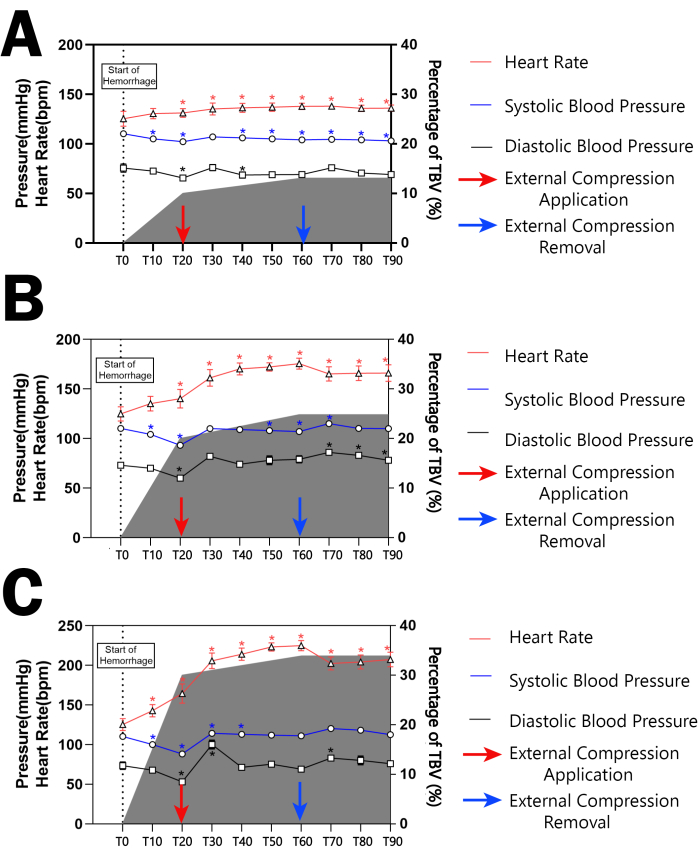
Figure 7: Hemodynamic responses during the experiment. (A-C) correspond to the three experimental groups subjected to different volumes of blood withdrawal. The graphs track the heart rate (red line), systolic blood pressure (blue line), and diastolic blood pressure (black line) over time, alongside the percentage of total blood volume (TBV) loss (shaded area). The data show the temporal progression of heart rate and blood pressure from the start of hemorrhage (T0) through to 90 min post-hemorrhage (T90). * indicates that the hemodynamic parameters at each time point have a statistically significant difference compared to T0 within each group (p<0.05). All continuous data were expressed as mean ± standard deviation, and significance was set at 95% confidence intervals (C.I.s). Repeated Measures One-way analysis of variance (ANOVA) was employed to determine the effect of hemorrhage volume on hemodynamic parameters. Please click here to view a larger version of this figure.
Discussion
Axillary artery injury is the main cause of massive hemorrhage at the upper extremity junctional site1. For active bleeding control, manual compression, gauze tamponade, and hemostatic agents are commonly used methods in combat or pre-hospital settings8. While compression alone can temporarily control junctional hemorrhage, it may not completely occlude the axillary artery due to the flexibility of the scapula. Additionally, most local hemostatic agents are in the form of gauze and membrane sheets that require manual compression to hold them in place at the bleeding site9. Currently, there are no relevant clinical studies that have confirmed a reliable compression pressure when combined with gauze tamponade or hemostatic agents. This study developed a new hybrid quantitative evaluation model for upper extremity junctional hemorrhage in swine that offers the advantage of further development of pre-hospital hemostatic products.
When constructing a hemorrhagic shock model, the primary challenge is to accurately replicate the clinical scenario while ensuring high reproducibility and standardization10. Therefore, we have developed a hybrid quantitative evaluation model that offers several advantages over the traditional hemorrhagic shock model:
This study introduces a swine axillary artery injury model for the first time. In recent publications, the majority of research has utilized the femoral artery injury model to evaluate the efficacy of new hemostatic agents11,12,13,14,15. However, limited attention has been given to describing the feasibility of the axillary artery injury model. This is attributed to the challenges associated with performing an axillary artery injury in swine, arising from the complex anatomical features of the swine axilla. To minimize unintentional injury or bleeding, an ultrasound-guided axillary approach is preferred to locate the axillary artery before dissection. This choice is made because the vessels are situated deep between the superficial and deep pectoral muscles and are surrounded by an axillary sheath. Meanwhile, another challenge is to achieve a consistent 2 mm transverse incision in all animals through visual estimation.
The vascular blocking bands provide traction force to temporarily occlude blood flow in the injured axillary artery (open artery). In the current landscape of research, one encounters critical challenges when developing novel hemostatic materials for junctional hemorrhages. These challenges stem from deep and narrow wounds in junctional regions that result in rapid and pressurized blood flow. These dynamics significantly hinder the ability of hemostatic materials to absorb and manage interfacial fluid. Under conditions of massive hemorrhage, this is especially detrimental, as the high-pressure, rapidly flowing blood can wash away hemostatic agents and disrupt any inadequately formed clots. The study introduces the use of two vascular blocking bands that temporarily control the pressurized blood flow. This strategy enables the evaluation of the efficacy of hemostatic materials without the confounding effects of active hemorrhage preceding the surgical intervention to manage bleeding.
The compression pressure in the experiment can be set to be a specific value according to the needs of the experiment and kept relatively stable throughout the experiment. The use of an external compression device to apply adjustable pressure to the tamponade gauze is a valuable development in the field of junctional hemorrhage research. The specific compression pressure of the upper extremity junctional hemorrhage has not yet been clarified. According to the case report of a gunshot wound, abdominal aortic and junctional tourniquet can be used in the axilla, but the required compression pressure was not clearly stated16. Johnson et al.5 verified the effectiveness and safety of Sam junctional tourniquet in the groin and axillary region, indicating occlusion of the axillary artery requires an average of 739 mmHg. In 2022, Avital et al.17 documented a case where a manual pressure points technique was utilized to control axillary hemorrhage during a 21 min flight transportation, following the repeated failure of a hemostatic bandage. This case report confirmed that the pressure points technique can successfully halt bleeding in the axillary junctional region and can be sustained over an extended period. However, the application of pressure was performed by an aeromedical evacuation medic, whose prolonged exertion led to fatigue, thereby compromising the ability to maintain consistent pressure throughout the procedure. In this model, the gauze tamponade and local compression were used simultaneously. We did not seek to block the axillary artery flow completely but used the method of gauze tamponade combined with external compression to achieve local hemostasis. The results indicate that only 210 mmHg of external pressure was required to effectively stop bleeding when combined with gauze tamponade. Additionally, the employment of a mechanical arm ensures a constant and unaltered pressure at the pressure point, thereby augmenting the effectiveness of this hemorrhage control strategy. For further research, the compression pressure of this device can be adjusted and customized based on the tamponade material. If various newly developed pressure-required hemostatic dressings need to be tested for their efficiency, the pressure can be adjusted to accommodate the experimental settings. For example, if the pressure is set to a specific value, it is possible to determine which hemostatic dressing is more effective by comparing the time to hemostasis and blood loss under compression.
Class I-III shock was induced by combining VCH with uncontrolled hemorrhage, accurately simulating the clinical scenario of axillary artery penetration injury while adjusting the total blood loss to the required range. The hemorrhagic shock model can be categorized into three types: volume-controlled, pressure-controlled, and uncontrolled. The volume-controlled hemorrhagic shock model simulates the process of hemorrhagic shock by releasing a certain proportion of blood based on the effective circulating volume over a set period. This model has advantages in evaluating hemodynamics (such as compensatory mechanisms), but it is difficult to control the degree of changes in blood pressure (such as hypotension). It has been found that rapid blood flow followed by slow exsanguination induces a more intense physiological response (heart rate, serum lactic acid, and amount of resuscitation fluid required) than constant exsanguination (traditional methods), and more realistically simulates the process of hemorrhagic shock. There are many ways to establish the model of uncontrolled hemorrhagic shock. The commonly used models of uncontrolled hemorrhagic shock include liver or spleen tear hemorrhage model, aortic and femoral artery injury hemorrhage model, etc.18,19,20,21,22. This hemorrhagic shock model is very close to the clinical situation of patients with trauma or severe bleeding and is widely used, especially in the study of fluid resuscitation of hemorrhagic shock. In this study, the blood drawing from the femoral artery of 10%, 20%, and 30% TBV combined with the fixed pressure compression of the mechanical arm induced Grade I-III hemorrhagic shock. Hemodynamic parameters were monitored every 10 min during the whole process to ensure the stability of the model.
This model has several significant limitations, including the aspect of uncontrolled hemorrhage. A major constraint is the precision required for the 2 mm transverse incision in the axillary artery. While our data show a narrow standard error for blood loss under compression, variability in incision size and, consequently, in uncontrolled hemorrhage may increase when different individuals perform the procedure. Another limitation is the anesthesia and pain management of the swine used in the experiment. Given the extensive surgical procedures involved in the surgical preparation phase of this model, managing analgesia and anesthesia depth is crucial. It is important to closely monitor for signs of pain and provide appropriate treatment during tracheostomy, cannulation, and axillary artery injury.
Conclusion
In this study, we introduced a novel hybrid quantitative evaluation model for upper extremity junctional hemorrhage in swine, marking a significant advancement in the development of pre-hospital hemostatic products. The utilization of a swine axillary artery injury model, paired with the implementation of vascular blocking bands and an external compression device, addresses critical challenges in the control of junctional hemorrhages, such as achieving consistent and controlled pressure to enhance hemostatic efficacy. Our findings demonstrate that a combination of gauze tamponade with a precisely controlled external pressure of 210 mmHg, further supported by the constant force provided by a mechanical arm, effectively stops bleeding. This approach not only provides a practical solution for managing axillary artery injuries but also sets the stage for future research on adjustable pressure mechanisms for various hemostatic dressings. This enhances the clinical relevance and applicability of the findings. These results have significant potential to improve human trauma care, especially in pre-hospital settings.
Disclosures
The authors declared that no competing conflicts of interest exist.
Acknowledgements
The authors would like to express their gratitude to Li Yang for designing the study's concept, Zhao Dongchu and Guo Yong for conducting the surgical procedures, Zhao Dongchu for data analysis and manuscript preparation, and Zhang Lianyang for supervising the research project. This work was supported by the Chongqing Municipal Health and Family Planning Commission and Science and Technology Commission Medical Scientific Research Project (No. 2024MSXM084), Construction of Key Clinical Specialties in the Military and Clinical Medical Research on Critical and Severe Diseases in Chongqing City.
Materials
| Name | Company | Catalog Number | Comments |
| Data Acquisition Software MCC DAQami | Measurement Computing Corporation | 4.2.1f0 | Advanced data acquisition and monitoring software designed to provide an intuitive and efficient user experience, developed by Measurement Computing Corporation based in Norton, MA, USA. |
| DF9-40 Flexible Film Pressure Sensor | Chengke Electronics | 500g | A flexible film pressure sensor designed for applications requiring force measurement |
| DuPont Wire, Data Acquisition Card | J.X. | CT USB-1208LS | A data acquisition card equipped with DuPont connectors for interface and signal processing tasks |
| Single Channel Signal Conditioning Conversion Module | Risym | IMS-C04A | A module used for conditioning and converting signals in a single channel setup |
References
- Morrison, J. J., Rasmussen, T. E. Noncompressible torso hemorrhage: a review with contemporary definitions and management strategies. Surg Clin North Am. 92 (4), 843-858 (2012).
- Sauaia, A., et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 38 (2), 185-193 (1995).
- van Oostendorp, S. E., Tan, E. C. T. H., Geeraedts, L. M. G. Prehospital control of life-threatening truncal and junctional haemorrhage is the ultimate challenge in optimizing trauma care; a review of treatment options and their applicability in the civilian trauma setting. Scand J Trauma Resusc Emerg Med. 24 (1), 110 (2016).
- Spiegel, S., Baker, A. M. . EMS junctional hemorrhage control. , (2023).
- Johnson, J. E., Sims, K., Hamilton, D. J., Kragh, J. F. Safety and effectiveness evidence of SAM(r) junctional tourniquet to control inguinal hemorrhage in a perfused cadaver model. J Spec Oper Med. 14 (2), 21-25 (2014).
- Coakley, T. A., Devlin, J. J., Kircher, S. S., Johnson, A. S. Development of a ballistic model of combat groin injury. J Trauma Acute Care Surg. 72 (1), 206-210 (2012).
- Zhu, Q., et al. Body mass index and waist-to-hip ratio misclassification of overweight and obesity in Chinese military personnel. J Physiol Anthropol. 39 (1), 24 (2020).
- La Monaca, G., et al. Hemorrhagic complications in implant surgery: a scoping review on etiology, prevention, and management. J Oral Implantol. 49 (4), 414-427 (2023).
- Peng, H. T. Hemostatic agents for prehospital hemorrhage control: a narrative review. Mil Med Res. 7 (1), 13 (2020).
- Fülöp, A., Turóczi, Z., Garbaisz, D., Harsányi, L., Szijártó, A. Experimental models of hemorrhagic shock: a review. Eur Surg Res. 50 (2), 57-70 (2013).
- Kheirabadi, B. S., et al. Comparison of new hemostatic granules/powders with currently deployed hemostatic products in a lethal model of extremity arterial hemorrhage in swine. J Trauma. 66 (2), 316-326 (2009).
- Kheirabadi, B. S., et al. Determination of efficacy of new hemostatic dressings in a model of extremity arterial hemorrhage in swine. J Trauma. 67 (3), 450-459 (2009).
- Wang, Y. H., et al. Evaluation of chitosan-based dressings in a swine model of artery-injury-related shock. Sci Rep. 9 (1), 14608 (2019).
- Angus, A. A., et al. Testing and evaluation of a novel hemostatic matrix in a swine junctional hemorrhage model. J Surg Res. 291, 452-458 (2023).
- Landers, G. D., et al. Efficacy of hemostatic gauzes in a swine model of prolonged field care with limb movement. Mil Med. 186 (Suppl 1), 384-390 (2021).
- Croushorn, J., McLester, J., Thomas, G., McCord, S. R. Abdominal aortic tourniquet controls junctional hemorrhage from a gunshot wound of the axilla. J Spec Oper Med. 13 (3), 1-4 (2013).
- Holcomb, J. B., et al. Effect of dry fibrin sealant dressings versus gauze packing on blood loss in grade V liver injuries in resuscitated swine. J Trauma. 46 (1), 49-57 (1999).
- Avital, G., et al. Pressure points technique for traumatic proximal axillary artery hemorrhage: a case report. Prehosp Disaster Med. , 1-4 (2022).
- Bickell, W. H., Bruttig, S. P., Wade, C. E. Hemodynamic response to abdominal aortotomy in the anesthetized swine. Circ Shock. 28 (4), 321-332 (1989).
- Matsuoka, T., Hildreth, J., Wisner, D. H. Liver injury as a model of uncontrolled hemorrhagic shock: resuscitation with different hypertonic regimens. J Trauma. 39 (4), 674-680 (1995).
- Krausz, M. M., Horn, Y., Gross, D. The combined effect of small volume hypertonic saline and normal saline solutions in uncontrolled hemorrhagic shock. Surg Gynecol Obstet. 174 (5), 363-368 (1992).
- Capone, A., Safar, P., Stezoski, S. W., Peitzman, A., Tisherman, S. Uncontrolled hemorrhagic shock outcome model in rats. Resuscitation. 29 (2), 143-152 (1995).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved