Lead Analysis of Soil Using Atomic Absorption Spectroscopy
Overview
Source: Laboratories of Margaret Workman and Kimberly Frye - Depaul University
Lead occurs naturally in soil, in levels ranging from 10-50 ppm. However, with the widespread use of lead in paint and gasoline in addition to contamination by industry, urban soils often have concentrations of lead significantly greater than background levels – up to 10,000 ppm in some places. Ongoing problems arise from the fact that lead does not biodegrade, and instead remains in the soil.
Serious health risks are associated with lead poisoning, where children are particularly at risk. Millions of children in the U.S. are exposed to soil containing lead. This exposure can cause developmental and behavioral problems in children. These problems include learning disabilities, inattention, delayed growth, and brain damage. The Environmental Protection Agency has set a standard for lead in soil at 400 ppm for play areas and 1,200 ppm for non-play areas.
Lead is also of concern in soil, when it’s used for gardening. Plants take up lead from the soil. Therefore, vegetables or herbs grown in contaminated soil can lead to lead poisoning. In addition, contaminated soil particles can be breathed in while gardening or brought into the house on clothing and footwear. It is recommended that soils with lead levels greater than 400 ppm should not be used for gardening. It is further recommended that soil with lead levels between 100 and 400 ppm not be used for leafy vegetables or herbs, because lead can be stored in the leaves. On a similar note, root vegetables should not be grown in this soil, because lead can also accumulate in plant roots.
Procedure
1. Soil Collection and Preparation
- In undisturbed areas, collect soil from the upper 1-2 inches of the soil. If sampling vegetable gardens, collect 6-inch deep samples. Use a soil auger to collect a 1-inch diameter soil core from sample area.
- Mix the sample thoroughly by shaking for 2 min and sieve using a USS #10 sieve.
- Dry the soil in a 40 °C oven for 24 h.
2. Sample Digestion
- Using an analytical balance, weigh out 1 g of the soil sampl
Results
The software creates the calibration curve and automatically determines the concentration of the Pb in the samples (Figure 2).
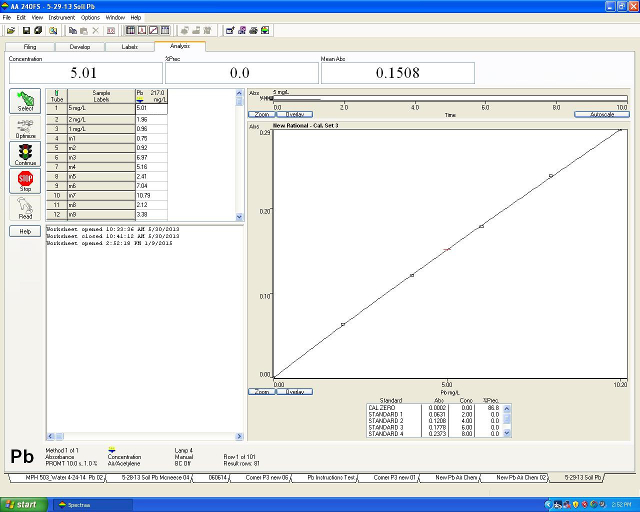
Figure 2. The calibration curve and the concentration of the Pb in the samples automatically determined by the software.
The values given on the worksheet are mg/L of Pb in the sample solution. Addi
Application and Summary
Atomic Absorption Spectrometry is a useful technique to analyze a wide range of environmental samples (e.g., water, soil, sludge, and sediment) for a large number of elements (e.g., heavy metals). This experiment highlights the use of flame AAS to determine the Pb content in soil. However, it could also be used to measure concentrations of Cu, Fe, Mn, K, Na, Mg, and Zn in soils.
Zinc is an important micronutrient and is needed for protein synthesis. Zn helps regulate the expr
References
- Robinson, J.W., Skelly Frame, E.M., Frame II, G.M. Undergraduate Instrumental Analysis. 6th Ed. Marcel Dekker, New York (2005).
- United States Environmental Protection Agency. “Lead based paint poisoning prevention in certain residential structures.” CFR 40 Part 745. http://www.ecfr.gov. (2015).
Skip to...
Videos from this collection:

Now Playing
Lead Analysis of Soil Using Atomic Absorption Spectroscopy
Environmental Science
125.9K Views

Tree Identification: How To Use a Dichotomous Key
Environmental Science
81.4K Views

Tree Survey: Point-Centered Quarter Sampling Method
Environmental Science
49.5K Views

Using GIS to Investigate Urban Forestry
Environmental Science
12.7K Views

Proton Exchange Membrane Fuel Cells
Environmental Science
22.2K Views

Biofuels: Producing Ethanol from Cellulosic Material
Environmental Science
53.5K Views

Testing For Genetically Modified Foods
Environmental Science
90.1K Views

Turbidity and Total Solids in Surface Water
Environmental Science
35.9K Views

Dissolved Oxygen in Surface Water
Environmental Science
56.0K Views

Nutrients in Aquatic Ecosystems
Environmental Science
39.0K Views

Measuring Tropospheric Ozone
Environmental Science
26.5K Views

Determination Of NOx in Automobile Exhaust Using UV-VIS Spectroscopy
Environmental Science
30.3K Views

Carbon and Nitrogen Analysis of Environmental Samples
Environmental Science
29.6K Views

Soil Nutrient Analysis: Nitrogen, Phosphorus, and Potassium
Environmental Science
216.2K Views

Analysis of Earthworm Populations in Soil
Environmental Science
16.6K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved
