For gas-phase equilibria, changes in the concentrations of reactants and products can occur with altered volume and pressure. The partial pressure, P, of an ideal gas is proportional to its molar concentration, M.
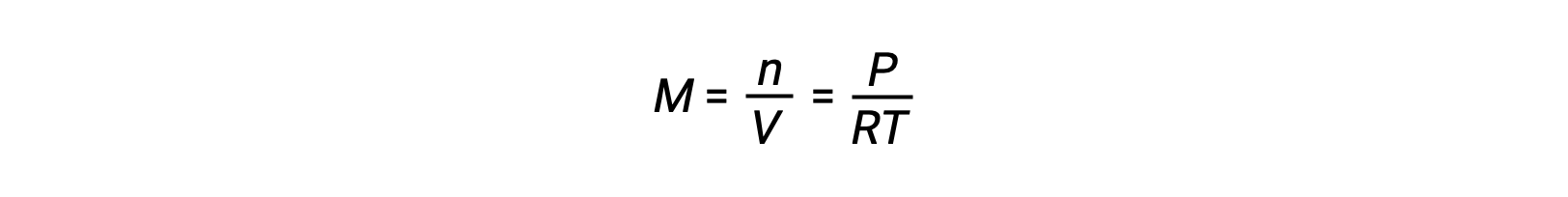
So changes in the partial pressures of any reactant or product are essentially changes in concentrations; therefore, these changes yield the same effects on equilibria. Aside from adding or removing reactants or products, the pressures (concentrations) of species in a gas-phase equilibrium can also be changed by changing the volume occupied by the system. Since all species of a gas-phase equilibrium occupy the same volume, a given change in volume will cause the same change in concentration for both reactants and products. In order to discern what shift, if any, this type of stress will induce, the stoichiometry of the reaction must be considered.
At equilibrium, the reaction N2 (g) + O2 (g) ⇌ 2 NO (g) is described by the reaction quotient
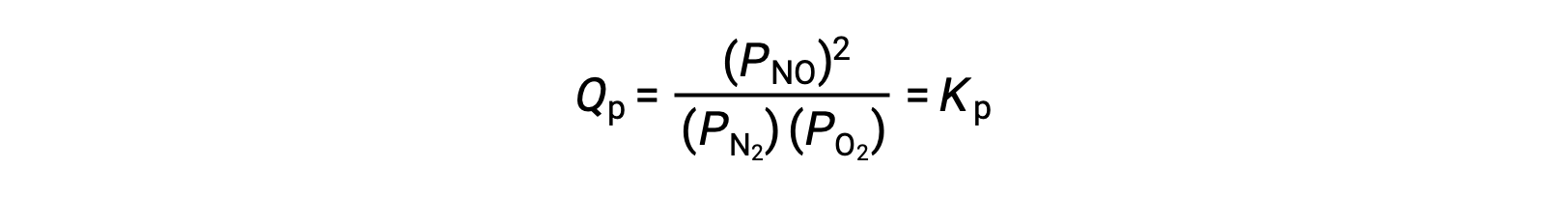
If the volume occupied by an equilibrium mixture of these species is decreased by a factor of 3, the partial pressures of all three species will be increased by a factor of 3:
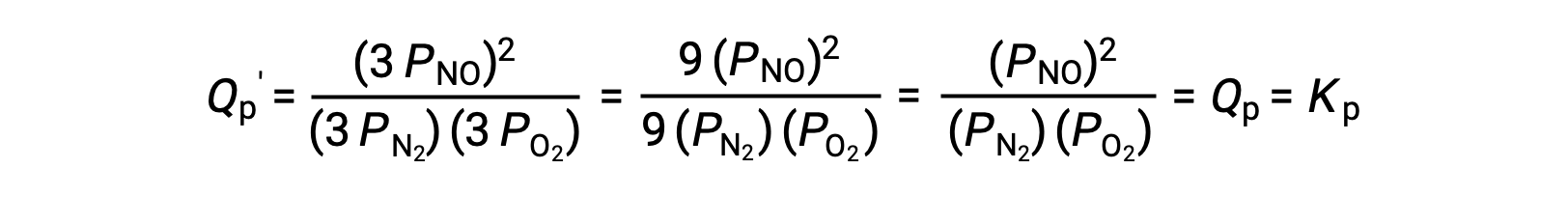
And so, changing the volume of this gas-phase equilibrium mixture does not result in a shift of the equilibrium.
A similar treatment of a different system, 2 SO2 (g) + O2 (g) ⇌ 2 SO3 (g), however, yields a different result:
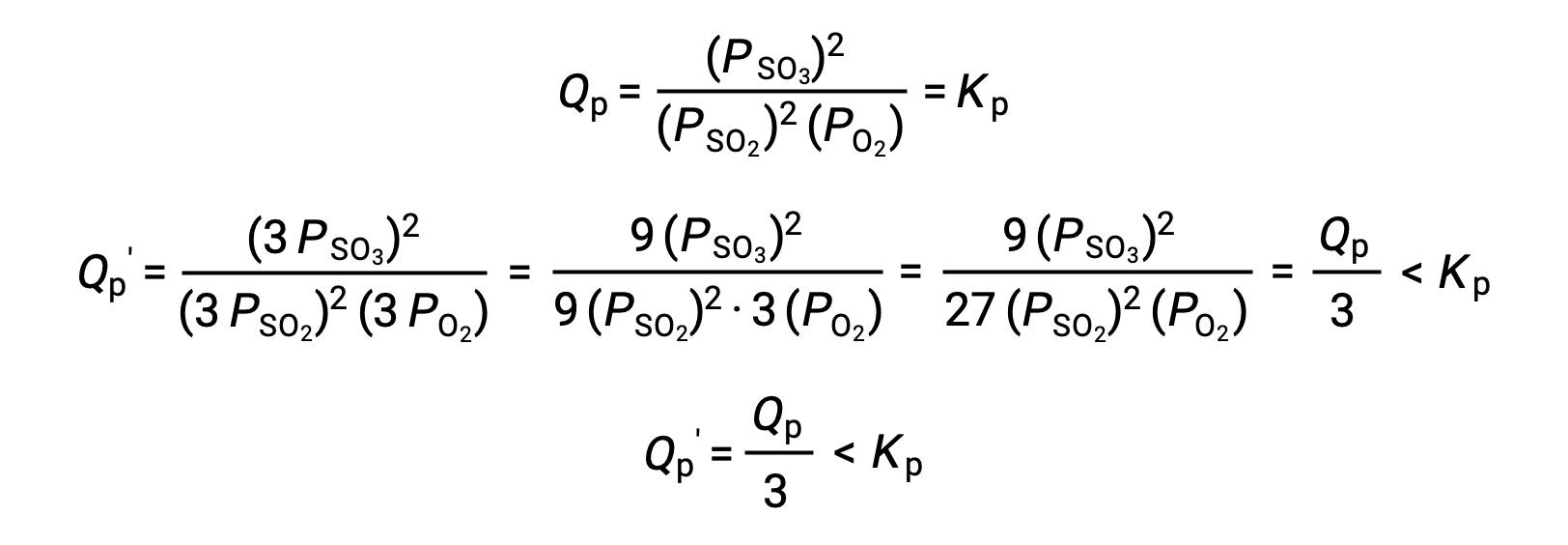
In this case, the change in volume results in a reaction quotient smaller than the equilibrium constant, and so the equilibrium will shift right.
These results illustrate the relationship between the stoichiometry of a gas-phase equilibrium and the effect of a volume-induced pressure (concentration) change. If the total molar amounts of reactants and products are equal, as in the first example, a change in volume does not shift the equilibrium. If the molar amounts of reactants and products are different, a change in volume will shift the equilibrium in a direction that better “accommodates” the volume change. In the second example, three moles of reactant (SO2 and O2) yield two moles of product (SO3), and so decreasing the system volume causes the equilibrium to shift right since the forward reaction produces less gas (2 mol) than the reverse reaction (3 mol). Conversely, increasing the volume of this equilibrium system would result in a shift towards reactants.
This text has been adapted from Openstax, Chemistry 2e, Section 13.3 Shifting Equilibria: LeChatelier’s Principle.
Aus Kapitel 14:

Now Playing
14.8 : Le Chatelier's Principle: Changing Volume (Pressure)
Chemisches Gleichgewicht
33.2K Ansichten

14.1 : Fließgleichgewicht
Chemisches Gleichgewicht
48.5K Ansichten

14.2 : Die Gleichgewichtskonstante
Chemisches Gleichgewicht
45.0K Ansichten

14.3 : Homogene Gleichgewichte für gasförmige Reaktionen
Chemisches Gleichgewicht
23.2K Ansichten

14.4 : Berechnung der Gleichgewichtskonstante
Chemisches Gleichgewicht
29.8K Ansichten

14.5 : Reaktionsquotient
Chemisches Gleichgewicht
47.1K Ansichten

14.6 : Berechnung von Gleichgewichtskonzentrationen
Chemisches Gleichgewicht
46.0K Ansichten

14.7 : Das Prinzip von Le Chatelier: Veränderte Konzentration
Chemisches Gleichgewicht
56.3K Ansichten

14.9 : Das Prinzip von Le Chatelier: Temperaturwechsel
Chemisches Gleichgewicht
28.2K Ansichten

14.10 : Die kleine x Annahme
Chemisches Gleichgewicht
45.3K Ansichten
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten