Zum Anzeigen dieser Inhalte ist ein JoVE-Abonnement erforderlich. Melden Sie sich an oder starten Sie Ihre kostenlose Testversion.
Zebrafish Electrocardiography (ECG): A Minimally Invasive Assay to Evaluate Cardiac Function
In diesem Artikel
Overview
This video shows how to perform electrocardiography (ECG or EKG) measurements, a test which measures the electrical activity of the heart, on a zebrafish.
Protokoll
This protocol is an excerpt from Zhao et al, In Vivo Surface Electrocardiography for Adult Zebrafish, J. Vis. Exp. (2019).
All experiments in this study were conducted in accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal protocols in this study were approved by the UCLA Institutional Animal Care and Use Committee.
1. Anesthesia induction
- Prepare immersion anesthesia for pain control and fish immobilization to avoid motion artifacts during ECG data acquisition. Most laboratories use immersion tricaine (ethyl 3-aminobenzoate methanesulfonate, MS-222).
- To make the tricaine 0.4% stock solution, combine the following items in a screw-capped dark glass bottle: 400 mg of tricaine powder, 98 mL of double distilled water, and 2 mL of 1 M Tris (pH 9). Adjust to pH 7.0 using 1 N NaOH or 1 N HCl as needed.
- To make the tricaine final immersion solution, determine the minimum concentration that is appropriate for the zebrafish age, size, metabolic state, strain, disease model, scientific objectives, and procedural duration.
- Perform a tricaine concentration-response study, titrating up or down from the recommended concentration of 168 mg/L (or 0.0168%)9if necessary, to attain level 4 of anesthesia within 3 min with the fewest possible cardiorespiratory toxicities. For example, in this study, the immersion of wild-type AB zebrafish of 12-18 months of age into a 0.02-0.04% tricaine solution will induce level 4 of anesthesia within 3 min.
NOTE: At level 4 of anesthesia, equilibrium and muscle tone are completely lost and opercular movement rate is reduced. - If necessary, consult the veterinarian in the Institutional Animal Care and Use Committee (IACUC) for additional guidance on the appropriateness of the selection of anesthetic(s) and route of administration.
- Immerse an adult zebrafish into a dish containing tricaine solution of the lowest predetermined and IACUC-approved concentration (e.g., 0.02-0.04% in this study) to induce level 4 of anesthesia within 3 min (Figure 1).
- For survival ECG protocol, keep the ECG recording session as brief as possible (under 10 min). For brief ECG recording sessions lasting less than 15 min, anesthesia maintenance is not necessary.
- For long ECG recording sessions lasting hours, use a long-acting intramuscular paralytic and an oral perfusion system to provide ample hydration and oxygenation.
2. ECG lead placement
- Once the zebrafish maintains level 4 of anesthesia for 3 s, use a pair of blunt forceps to transfer the fish immediately onto the damp sponge slit with its ventral surface uppermost for placement of ECG lead electrodes (Figure 2).
- Gently insert the three ECG lead electrodes into the fish musculature to approximately 1 mm in depth to establish a bipolar lead in the frontal plane that parallels the left caudal-right cranial orientation of the cardiac main axis.
- Position the positive (red) electrode in the ventral midline at the level of the bulbus arteriosus, i.e., at 1-2 mm above an imaginary line connecting the two lower edges of the operculums (Figure 2A).
- Position the negative (black) electrode caudally and 0.5-1.0 mm left laterally to the positive electrode, at a distance greater than the maximal apicobasal length of the adult zebrafish ventricle (Figure 2A).
- Position the reference (green) electrode caudally, near the anal region.
NOTE: Since the cardiac main axis varies somewhat from fish to fish, to maximize the R and T wave amplitudes, adjust the lead positions by making only small, systematic changes through trial and error. For example, change one electrode (positive or negative), instead of both electrodes, at a time and make gradual changes in one specified direction before changing to another direction instead of making erratic changes in random directions.
3. ECG recording
- Open the ECG data acquisition program. Select a desired setting from the drop-down menus for range, low pass, and high pass. For example, the following setting in the in vivo ECG recording system used in this experiment yields consistent, satisfactory signal-to-noise ratio for a normal adult zebrafish: range "2 mV", low pass "120 Hz", and high pass "0.03 s".
- Press Start to start continuous gap-free ECG recording at a sampling rate of 1 kHz.
- To optimize lead positioning for maximal signal-to-noise ratio, press Stop to stop ECG recording and review the ECG trace soon after the very first recording attempt for each heart. To diagnose that an adult zebrafish ECG is normal, confirm that all of the following four validating criteria are satisfied (Figure 3):
- Criterion 1: Ensure that all ECG waveforms (P, QRS, and T) are distinct and readily visible.
- Criterion 2: Ensure that the P wave is positive.
- Criterion 3: Ensure that the net QRS complex is positive (i.e. the R wave amplitude is larger than the sum of Q and S wave amplitudes).
- Criterion 4: Ensure that the T wave is positive.
- If a normal ECG is expected, reposition the electrodes (try the negative electrode first) if necessary, until all four validating criteria are satisfied.
- If a normal T wave is expected, but the T wave is too small, reposition the electrodes to maximize the T wave amplitude.
- Resume ECG recording after optimizing lead positioning. Save the ECG sweeps for subsequent analysis.
4. Recovery from anesthesia
- At the end of the ECG recording session, carefully remove the electrodes without injuring the fish. Transfer the fish to fresh, oxygenated fish water free of tricaine.
- To facilitate recovery from anesthesia, squirt water over the gills vigorously with a Pasteur pipette until the fish resumes regular gill movement or swimming.
- Monitor the fish for full recovery from anesthesia (typically 1-2 min), as indicated by the fish ability to swim upright for at least 5 s.
Ergebnisse
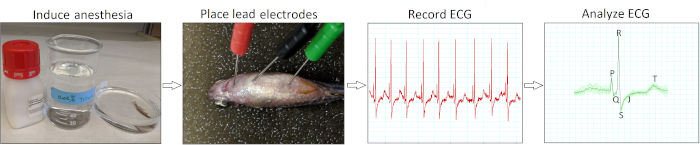
Figure 1: Minimally invasive in vivo ECG recording protocol. A schematic flow chart illustrates four critical action steps in conducting an in vivo ECG interrogation: induce anesthesia, place ECG lead electrodes, record ECG, and analyze the ECG recordings. Please click here to view a larger version of this figure.
Materialien
| Name | Company | Catalog Number | Comments |
| Culture dishes | Fisher Scientific | FB087571 | 100 mm x 20 mm |
| Dumont Forceps | Fine Sciense Tools | 11253-20 | 0.1 x 0.06 mm |
| FE136 Animal Bio Amp | AD Instruments | FE231 | |
| Iris Forceps | Fine Sciense Tools | 11064-07 | 0.6 x 0.5 mm |
| LabChart 8 Pro | AD Instruments | Software with ECG Module | |
| Needle electrodes for Animal Bio Amp | AD Instruments | MLA1213 | 29 gauge |
| Plastic Disposable Transfer Pipets | Fisher Scientific | 13-669-12 | 6 in., 1.2 mL |
| PowerLab 4/35 | AD Instruments | 4//35 | |
| Tricaine (Ethyl 3-aminobenzoate methanesulfonate) | Sigma | E10521-10G | MS-222 |
This article has been published
Video Coming Soon
Source: Zhao, Y., et al. In Vivo Surface Electrocardiography for Adult Zebrafish. J. Vis. Exp. (2019).
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten