Zum Anzeigen dieser Inhalte ist ein JoVE-Abonnement erforderlich. Melden Sie sich an oder starten Sie Ihre kostenlose Testversion.
Assessing Attractive or Repulsive Activity of Proteins Using Hippocampal Neurons
In diesem Artikel
Overview
This video demonstrates a method to assess protein activity using hippocampal neurons over patterned protein zones. Neurons exhibit repulsion in test protein zones and attachment in control protein zones, highlighting the effects of attractive or repulsive protein activity.
Protokoll
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparation of Matrices
- Boil 4-8 silicone matrices in a microwave or on a hot plate for 5 min and allow them to dry completely for 1 h under laminar flow (striped side up).
NOTE: The following procedures should be performed under laminar flow. - Blow compressed air or use transparent sticky tape to remove any dust from the striped side of the matrices. Keep the striped side clean so it can firmly adhere to the plate surface.
- Carefully place the matrices onto 6-cm plastic dishes by pressing with a finger (one matrix per dish; stripe side down). Avoid getting air bubbles between the matrix and the dish. Mark the location of the stripes on the bottom side of the culture dish.
- If the matrix fails to attach, repeat from step 1.2.
2. Stripe Generation and Laminin Coating for Neuron Culture
- Prepare fluorescently labeled recombinant protein (25 µl each for injection [step 2.2] or 100 µl each for the placement method [step 2.2.1]) by mixing the Fc-tagged protein (10-50 µg/ml) and a fluorescent dye-conjugated anti-human Fc antibody (1 to 3 ratio: 30-150 µg/ml) in phosphate buffered saline (PBS). Then, incubate the mixture for 30 min at RT.
- Using a 22-G syringe, inject 25 µl of the recombinant protein prepared in the previous step (2.1) through the small hole on the side of the matrix (arrows in Figure 1A, 1C, and 1E).
- Alternatively, place 100 µl of the recombinant protein into the top slit of the matrix and aspirate approximately half of the solution from the small hole (arrows in Figure 1A, 1C, and 1E), so that the recombinant protein remains in the striped regions.
- Incubate the dish for 30 min at 37 °C in a cell culture incubator.
- Place 300 µl of PBS into the top slit (Figure 1B) and aspirate from the small hole located on the side of the matrix (arrows in Figure 1A, C and E) to remove unattached recombinant proteins. Do not aspirate the PBS completely, because the proteins will dry. Repeat this step twice. (3 times total)
- Carefully remove the matrix and immediately place ~100 µl of control protein (10-50 µg/ml; Fc) to cover the entire striped area, creating an alternate coating. Then, incubate the dish for 30 min at 37 °C in the cell culture incubator.
- After washing the dish surface three times with PBS, coat it with ~100 µl of 20 µg/ml laminin in PBS.
NOTE: Laminin should be thawed on ice to prevent solidification. - Incubate the dish for 1 h at 37 °C in the cell culture incubator.
- Wash the dish surface three times with PBS and add culture medium. Place the coated dish with culture medium in a 5% CO2 incubator at 37 °C until use.
3. Culturing Hippocampal Neurons from E15.5 Mouse Embryos
- Euthanize the mother via cervical dislocation and isolate three E15.5 embryos.
- Cut the skin and skull sagittally at the midline of the head with scissors, and remove the brain using a small spoon. Transfer the brain carefully to a 60-mm Petri dish containing 3 ml of Hank's Balanced Salt Solution (HBSS).
- Using a scalpel, cut out the hemispheres including cortex, hippocampus and striatum (Figure 2A). Then, dissect the hippocampal region by carefully removing the meninges, and transfer the dissected hippocampal tissues to a 15-ml centrifuge tube containing 2 ml of HBSS solution, on ice (Figure 2B-C). Collect 6 hippocampi from 3 embryos in the tube, which are sufficient for culturing neurons in 8 dishes.
- Replace the HBSS solution with trypsin/ethylenediamine tetraacetic acid (trypsin/EDTA) solution (2 ml) and incubate the tube at 37 °C for 15 min in a water bath.
NOTE: Aspirate HBSS without centrifugation and do not decant the HBSS solution by tilting the tube. - Neutralize the trypsin activity by adding 500 µl of fetal bovine serum (FBS).
- Centrifuge the mixture at 100 x g for 5 min. Remove the supernatant and wash the pellet with 2 ml of culture medium. Repeat this step once more.
- Pipet the hippocampi up and down 10 times in a culture medium using a 1,000-µl tip with a large hole (cut-tip) to dissociate the tissue.
- Change to a regular (uncut) 1,000-µl tip and pipet the dissociated tissue up and down to obtain a single-cell suspension.
- Separate the single cells by passing them through an 80-100-µm mesh cell strainer.
- Centrifuge the filtered single-cell suspension at 150 x g for 5 min.
- Remove the supernatant by aspirating, and resuspend the pellet (neurons) in 2 ml of culture medium.
- Count the cells using a hemocytometer with trypan blue staining to determine the density and viability. Then, plate 10,000 neurons in 150 µl of culture medium for each set of stripes. The suspension should be carefully placed to cover the entire stripe region.
Ergebnisse
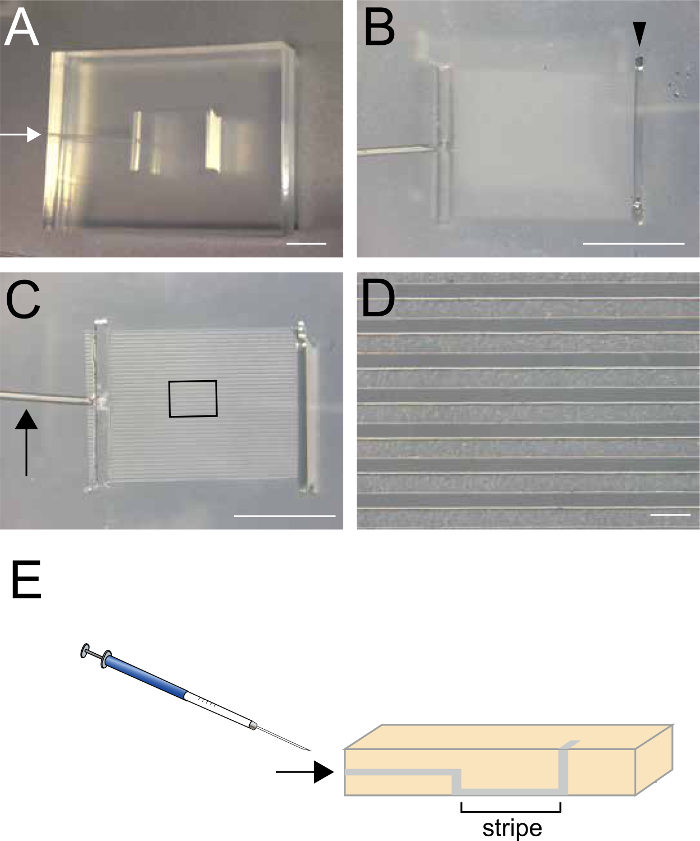
Figure 1. Silicon Matrix used to create the striped protein stamp. (A, B) Top view of the silicon matrix. The size of matrix is 30 mm x 25 mm x 5mm. White arrow in A indicates a small hole where the recombinant protein is injected (step 2.2). Arrowhead in B indicates a slit where the recombinant protein is alternatively applied (step 2.2.1). (C, D) Bottom view of the silicon matr...
Offenlegungen
Materialien
| Name | Company | Catalog Number | Comments |
| 15 mL centrifuge tube | Violamo | 1-3500-01 | |
| Alexa Fluor 488 Goat anti-human IgG antibody | Thermo Scientific | A11013 | |
| Alexa Fluor 594 Donkey anti-mouse IgG antibody | Thermo Scientific | A-21203 | Dilution 1/500 |
| B27 supplement | Thermo Scientific | 17504-044 | Dilution 1/50 |
| Bovine serum albumin | Sigma | 01-2030-2 | |
| Cell strainer 100 um | BD Falcon | 352360 | |
| Centrifugation machine | Kubota | 2410 | |
| DAKO pen | DAKO | S2002 | Alternative water-repellent pen may be used |
| Disposable scalpel | Feather | 2975#11 | |
| FBS | Thermo Scientific | 10437-028 | |
| Forceps No. 5 | Fine Science Tools | 11254-20 | |
| GlutaMAX | Thermo Scientific | 35050-061 | Dilution 1/200 |
| Hamilton Syringe | Hamilton | 805N | 22 gauge, 50 uL |
| HBSS | Thermo Scientific | 14170-112 | |
| Human IgG, Fc Fragment | Jackson | 009-000-008 | |
| Laminin | Thermo Scientific | 23017-015 | |
| Neurobasal | Thermo Scientific | 21103-049 | |
| PBS | Nacalai | 14249-24 | |
| Penicillin-Streptomycin | Thermo Scientific | 15070-063 | Dilution 1/100 |
| Plastic culture dish, 60 mm | Thermo Scientific | 150288 | |
| Silicone Matrices | Available and purchasable from Prof. Martin Bastmeyer (bastmeyer@kit.edu) | ||
| Stereo Microscope | Olympus | SZ61 | |
| Tip, 1000 uL | Watson | 125-1000S | |
| Transparent sticky tape | Tesa | 57315 | Alternative sticky tape may be used |
| Trypan blue, 0.4% | Bio-Rad | 145-0013 | |
| Trypsin/EDTA | Thermo Scientific | 25300-054 | |
| Culture medium | Neurobasal supplemented with B27, GlutaMAX and Penicillin-Streptomycin. |
This article has been published
Video Coming Soon
Source: Yamagishi, S. et al., Stripe Assay to Study the Attractive or Repulsive Activity of a Protein Substrate Using Dissociated Hippocampal Neurons. J. Vis. Exp. (2016)
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten