Zum Anzeigen dieser Inhalte ist ein JoVE-Abonnement erforderlich. Melden Sie sich an oder starten Sie Ihre kostenlose Testversion.
Assessing the Structural Integrity of the Dorsal Longitudinal Muscle Neuromuscular Junctions in Drosophila
In diesem Artikel
Overview
This video demonstrates a protocol for assessing the structural integrity of neuromuscular junctions in the dorsal longitudinal muscles of Drosophila. The procedure involves staining neurons and muscles with two distinct antibodies and comparing fluorescence patterns between mutant and wild-type flies. A reduction in neuronal staining in mutant flies indicates neurodegeneration, while the muscle structure remains intact.
Protokoll
1. Flash freezing and thorax bisection
- Before beginning the bisections, fill a Dewar flask with liquid nitrogen wearing proper cryo-protective gloves and safety glasses. Obtain a blade breaker, feather blades, one pair of fine forceps, ice, ice-cold 1x PBS, and cryogenic tweezers.
- Prepare an ice bucket to keep 1x PBS ice cold.
- Use the blade breaker to grab the feather blade at an angle and bend the blade in order to break off a small piece. The blade breaker can then lock the blade in position for use as a small scalpel.
NOTE: One blade should last for all groups. Switch if the blade breaks or becomes dull. - Add a clean pipette tip to the P200 and remove 1/5th of the tip to transport samples.
- Prepare a new microcentrifuge tube for each group and add 200 μL of 1x PBS to each tube. This second tube will be used to collect the final DLM preps.
- Remove all 1x PBS from tubes using a Pasteur pipette.
- Wearing proper protective equipment, submerge the tube into the liquid nitrogen flask for 10 s with the cryogenic tweezers.
NOTE: The tubes should be closed tightly to keep the tube from exploding. - Remove the tube from the liquid nitrogen and add approximately 300 μL of ice-cold 1x PBS to the samples with a Pasteur pipette. Keep the samples on ice.
- Add ice-cold 1x PBS to the 10 cm dissection dish coated with silicone elastomer and dispense the first thorax with the modified 200 μL pipette.
- Place the thorax ventral side up. In one hand use a dull pair of forceps to position the thorax and in the other use a fine pair of forceps to remove some of the thoracic ganglion to expose the midline of the thorax.
- Use the midline of the thorax as a guide to make a shallow cut through 1/3rd of the thorax with the blade.
- Remove the blade from the thorax and position the thorax at a 45° angle with the blunt forceps. Reinsert the blade and cut straight down the midline of the thorax. This will result in two hemithoraces.
- Take one hemithorax at a time and remove the excess tissue under DLM muscle fiber F (Figure 1B), the most ventral fiber. Use the blade to carefully make one or two cuts to remove the excess tissue without damaging the DLMs.
- Once isolated, transfer the hemithorax to the correct tube with 1x PBS.
- Repeat steps 1.6‒1.14 until 10 dissected hemithoraces per group are made.
2. Structural staining
- After bisecting the thorax samples, place the tissue in blocking buffer (1x PBS with 0.1% normal goat serum, and 0.2% Triton X-100 at pH 7.4) to permeabilize the tissue and prevent non-specific staining. Use a Pasteur pipette to remove excess 1x PBS and add 1.5 mL of blocking buffer to each tube. Block tissues for at least 1 h at 4 °C.
- Prepare the samples for structural staining using a fluorescently conjugated antibody, horseradish peroxidase 488 (anti-HRP-488) at a dilution of 1:200 and Phalloidin-647 at a dilution of 1:1000 in blocking buffer to stain motor neurons and muscle tissue, respectively. Make enough stain to have 150 μL per tube. Store the stain at 4 °C covered in foil or in a dark box until ready for staining.
- After blocking, remove the excess blocking buffer with a glass Pasteur pipette.
- Before dispensing the structural stain, vortex the stain. Add 150 μL of the stain to each tube. Place the samples in a dark box on the rotator at room temperature for 2 h.
- Remove the stain and wash the tissues four times in 1.5 mL of room temp 1x PBS with 0.3% Triton X-100 for 5 min on the rotator in a dark box. The samples are now ready to mount to a slide.
3. Mounting tissue
- After washing samples in PBST, prepare a microscope slide to mount tissue for staining. Prepare additional supplies including glass cover slips, a P200 pipette, 200 μL pipette tips, scissors, clear reinforcements, straight edge forceps, anti-fade fluorescent mounting media, nail polish, and a dark box to cover the slides.
- Label the slide to identify the samples and clean it with Kim wipes to remove any smudges.
- To ensure the cover slip does not damage the hemithorax samples, build a "bridge" using reinforcement labels. Cut one reinforcement label in half and place each half approximately 15 mm apart. This distance must be smaller than the width of the cover slip. Repeat this step four times to complete a "bridge" that is 5 labels high.
- Take the P200 pipette and modify a tip by cutting off 1/5th of the tip to transfer the samples to the slide. Samples should be transferred onto the slide in the center of the bridge.
- Take the edge of a lab wipe and remove any excess PBST. Using forceps, arrange the DLMs such that all samples are facing muscle side up and cuticle side down.
- Using a standard P200 pipette tip, apply 70 μL of mounting media to the slide, avoiding air bubbles. Dispense the media in a circular pattern inside the reinforcements starting from the outside into the center.
- Place a cover slip over the reinforcements.
- Use nail polish to coat the outside edges around the perimeter of the coverslip. Apply generously to form a complete seal of the tissue.
- Place the slide on a flat surface in the dark, allowing at least 10 min to dry and prevent photo-bleaching or loss of fluorescence. Slides may now be used for imaging immediately, or otherwise stored in a slide folder at -20 °C for later viewing.
Ergebnisse
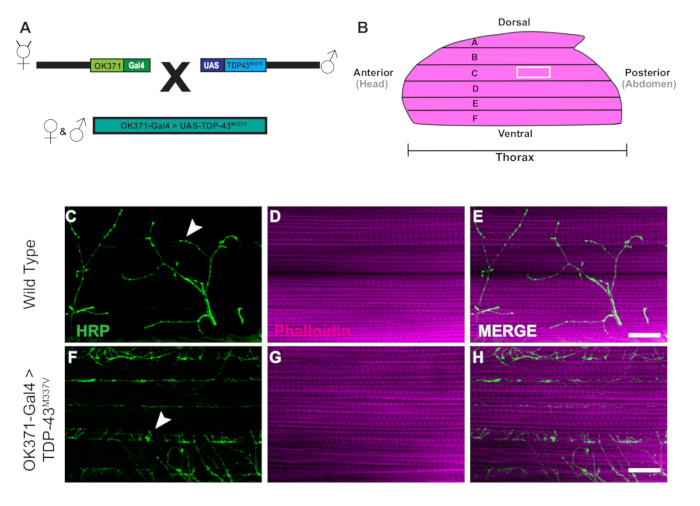
Figure 1: Progressive denervation of DLM synapses in a Drosophila model of ALS. (A) The generation of ALS transgenic flies expressing a human mutant form of Tar-Binding Protein of 43 kDa (TDP-43) are shown in the schematic. (B) The illustration depicts the shape and orientation of a hemithorax in an adult Drosophila. Using the protocol, we can o...
Offenlegungen
Materialien
| Name | Company | Catalog Number | Comments |
| 32% Formaldehyde | Electron Microscopy Sciences | 15714 | Tissue preservation |
| BenchRocker | Genesee Scientific | 31-302 | Rotating samples during staining |
| Blade Breaker | Fine Science Tools | 10053-09 | Used for holding feather blade |
| cover slips | Fisher Scientific | 12548A | For mounting tissue |
| cryogenic gloves | VWR | 97008-198 | protect hands from liquid nitrogen |
| cryogenic tweezers | VWR | 82027-432 | Hold 2.0 mL tube in liquid nitrogen |
| dewar flask-1900 mL | Thomas Scientific | 5028M54 | Hold liquid nitrogen |
| Feather Blades | Electron Microscopy Sciences | 72002-01 | Scalpel Blades |
| Fine Forecps x 2 | Fine Science Tools | 11252-20 | One fine pair for Clearing midline of thorax. The other pair can be dulled using a sharpening stone. |
| FITC-conjugated anti HRP | Jackson Laboratories | 123-545-021 | Stains Motor Neurons. Used at 1:100 concentration |
| freezer box (Black) | Fisher Scientific | 14100F | Protects samples from light |
| glass pasteur pipettes | VWR | 14637-010 | Used to transfer samples |
| glass slides | Fisher Scientific | 12550143 | For mounting tissue |
| mounting media (vectashield) anti- fade | VWR | 101098-042 | Mounting media retains fluorescent signaling |
| nail polish | Electron Microscopy Sciences | 72180 | Seals microscope slides |
| normal goat serum | Fisher Scientific | PCN5000 | Prevents non-specific binding of antibodies |
| paint brush | Genesee Scientific | 59-204 | Transferring flies |
| PBS | Fisher Scientific | 10-010-023 | Saline solution for dissecting and staining |
| Phalloidin 647 | Abcam | AB176759 | Stains F-Actin in muscle Tissue. Used at 1:1000 concentration |
| plastic petri dish (100 mm) | VWR | 25373-100 | Dissection dish |
| reinforcement labels | W.B. Mason | AVE05722 | Provides support for glass coverslip over the mounted tissue |
| sharpening block | Grainger | 1RDF5 | Keeping fine forceps sharp and also dulling separate pair |
| slide folder | VWR | 10126-326 | Sample storage |
| standard office scissors | W.B. Mason | ACM40618 | Cutting reinforcement labels |
| Sylgard 184 | Electron Microscopy Sciences | 24236-10 | Coating for dissection dish |
| Triton-X-100 | Electron Microscopy Sciences | 22140 | Helps to permeabilize tissue |
| Vannas Disssection Sissors | Fine Science Tools | 1500-00 | Ued for removing fly legs and making an incision on thorax |
This article has been published
Video Coming Soon
Source: Sidisky, J. M., et al. Visualizing Synaptic Degeneration in Adult Drosophila in Association with Neurodegeneration. J. Vis. Exp. (2020).
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten